Interactions in saccharide/cation/water systems: Insights from density functional theory
Texte intégral
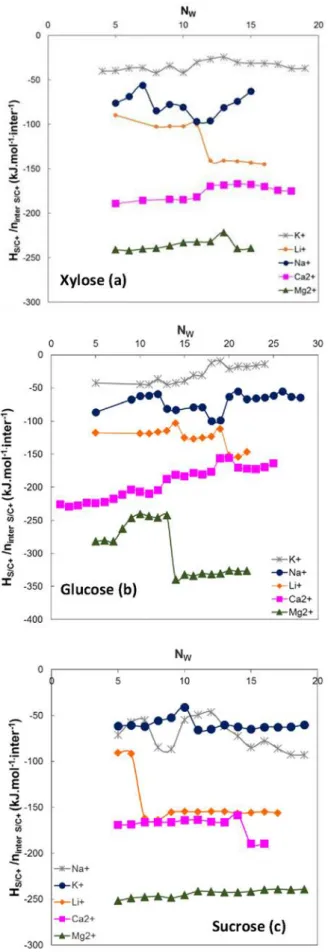
Figure

Documents relatifs
We turn now to the results obtained with the various DFT xc functionals and first consider 共i兲 if the DFT xc func- tionals tested are able to predict the correct energetic order- ing
In summary, we have developed a novel methodology to efficiently catalyze cyanation of imines under mild conditions using azaphosphatranes as catalysts. In most cases, high
In region I, the time shift of the photoelectron signal corre- sponding to ionization of the ππ*-state to the ionic ground state could arise from: (a) large amplitude motion,
PEKs with phenyl and 3-methylphenyl pendant groups were found to have controlled sulfonation sites with single substituted sulfonic acid per repeated unit via a postsulfonation
ADVANTAGES OF A BPM-BASED APPROACH During our work, we identified the following requirements for an experiment description and its execution: descrip- tiveness, modularity,
effects of feed and coolant inlet temperatures, feed flow rate and their interactions on the PGMD 98.. process performance experimentally
These calculations determined the molecular reorientation time averaged over all water molecules present in the fi rst hydration shell and consistent with the observable probed in
[ 21 ] In summary, the deep conductivity profile obtained beneath the Western Alps in the depth range 200 – 1000 km with laboratory studies agrees with a cold mantle where water