HAL Id: dumas-01747296
https://dumas.ccsd.cnrs.fr/dumas-01747296
Submitted on 5 Jun 2018HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
et la cicatrisation muqueuse au cours des MICI traitées
par combothérapie ?
Juliette Viaud
To cite this version:
Juliette Viaud. Le taux de 6-TGN est-il associé à la rémission clinique et la cicatrisation muqueuse au cours des MICI traitées par combothérapie ?. Sciences du Vivant [q-bio]. 2016. �dumas-01747296�
THESE D’EXERCICE/ UNIVERSITE DE RENNES 1 FACULTÉ DE MÉDECINE
sous le sceau de l’Université Européenne de Bretagne
THÈSE EN VUE DU
DIPLÔME D'ÉTAT DE DOCTEUR EN MÉDECINE
présentée par
Juliette VIAUD
né(e) le 23 septembre 1988 à Lisieux
Le taux de 6-TGN
est-il associé à la
rémission clinique et
la cicatrisation
muqueuse au cours
des MICI traitées par
combothérapie ?
Thèse soutenue à Rennes le 24 juin 2016
devant le jury composé de :
Jean-François BRETAGNE
PU-PH, CHU Rennes / président
Laurent SIPROUDHIS
PU-PH, CHU Rennes / Juge
Marie-Clémence VERDIER
MCU-PH, CHU Rennes/ Juge
Guillaume BOUGUEN
PHU, CHU Rennes/ directeur de thèse
LISTE DES ENSEIGNANTS
PROFESSEURS DES UNIVERSITES –PRATICIENS HOSPITALIERS
ANNE-GALIBERT Marie Dominique Biochimie et biologie moléculaire
BELAUD-ROTUREAU Marc-Antoine Histologie; embryologie et cytogénétique
BELLISSANT Eric Pharmacologie fondamentale; pharmacologie clinique; addictologie BELLOU Abdelouahab Thérapeutique; médecine d'urgence; addictologie
BELOEIL Hélène Anesthésiologie-réanimation; médecine d'urgence
BENDAVID Claude Biochimie et biologie moléculaire
BENSALAH Karim Urologie
BEUCHEE Alain Pédiatrie
BONAN Isabelle Médecine physique et de réadaptation
BONNET Fabrice Endocrinologie, diabète et maladies métaboliques; gynécologie médicale
BOUDJEMA Karim Chirurgie générale
BOUGET Jacques Thérapeutique; médecine d'urgence; addictologie
BOURGUET Patrick
Professeur des Universités en surnombre Biophysique et médecine nucléaire
BRASSIER Gilles Neurochirurgie
BRETAGNE Jean-François Gastroentérologie; hépatologie; addictologie BRISSOT Pierre
Professeur des Universités en surnombre Gastroentérologie; hépatologie; addictologie
CARRE François Physiologie
CATROS Véronique Biologie cellulaire
CHALES Gérard
Professeur des Universités émérite Rhumatologie
CORBINEAU Hervé Chirurgie thoracique et cardiovasculaire
CUGGIA Marc Biostatistiques, informatique médicale et technologies de communication
DAUBERT Jean-Claude
Professeur des Universités émérite Cardiologie
DAVID Véronique Biochimie et biologie moléculaire
DAYAN Jacques
Professeur des Universités associé Pédopsychiatrie; addictologie
DE CREVOISIER Renaud Cancérologie; radiothérapie
DECAUX Olivier Médecine interne; gériatrie et biologie du vieillissement; addictologie
DELAVAL Philippe Pneumologie; addictologie
DESRUES Benoît Pneumologie; addictologie
DEUGNIER Yves
Professeur des Universités en surnombre Gastroentérologie; hépatologie; addictologie
DONAL Erwan Cardiologie
DRAPIER Dominique Psychiatrie d'adultes; addictologie
DUPUY Alain Dermato-vénéréologie
ECOFFEY Claude Anesthésiologie-réanimation; médecine d'urgence
EDAN Gilles Neurologie
FERRE Jean Christophe Radiologie et imagerie Médecine
FEST Thierry Hématologie; transfusion
FLECHER Erwan Chirurgie thoracique et cardiovasculaire
FREMOND Benjamin Chirurgie infantile
GANDEMER Virginie Pédiatrie
GANDON Yves Radiologie et imagerie Médecine
GANGNEUX Jean-Pierre Parasitologie et mycologie
GARIN Etienne Biophysique et médecine nucléaire
GAUVRIT Jean-Yves Radiologie et imagerie Médecine
GODEY Benoit Oto-rhino-laryngologie
GUIGUEN Claude
Professeur des Universités émérite Parasitologie et mycologie
GUILLÉ François Urologie
GUYADER Dominique Gastroentérologie; hépatologie; addictologie
HOUOT Roch Hématologie; transfusion
HUGÉ Sandrine
Professeur des Universités associé Médecine générale HUSSON Jean-Louis
Professeur des Universités en surnombre Chirurgie orthopédique et traumatologique
JEGO Patrick Médecine interne; gériatrie et biologie du vieillissement; addictologie
JEGOUX Franck Oto-rhino-laryngologie
JOUNEAU Stéphane Pneumologie; addictologie
KAYAL Samer Bactériologie-virologie; hygiène hospitalière
KERBRAT Pierre Cancérologie; radiothérapie
LAMY DE LA CHAPELLE Thierry Hématologie; transfusion
LAVIOLLE Bruno Pharmacologie fondamentale; pharmacologie clinique; addictologie
LAVOUE Vincent Gynécologie-obstétrique; gynécologie médicale
LE BRETON Hervé Cardiologie
LE GUEUT Maryannick Médecine légale et droit de la santé
LE TULZO Yves Réanimation; médecine d'urgence
LECLERCQ Christophe Cardiologie
LEGUERRIER Alain Chirurgie thoracique et cardiovasculaire
LEJEUNE Florence Biophysique et médecine nucléaire
LEVEQUE Jean Gynécologie-obstétrique; gynécologie médicale
LIEVRE Astrid Gastroentérologie; hépatologie; addictologie
MABO Philippe Cardiologie
MEUNIER Bernard Chirurgie digestive
MICHELET Christian Maladies infectieuses; maladies tropicales
MOIRAND Romain Gastroentérologie; hépatologie; addictologie
MORANDI Xavier Anatomie
MORTEMOUSQUE Bruno Ophtalmologie
MOSSER Jean Biochimie et biologie moléculaire
MOULINOUX Jacques Biologie cellulaire
MOURIAUX Frédéric Ophtalmologie
ODENT Sylvie Génétique
OGER Emmanuel Pharmacologie fondamentale; pharmacologie clinique; addictologie
PERDRIGER Aleth Rhumatologie
PLADYS Patrick Pédiatrie
POULAIN Patrice Gynécologie-obstétrique; gynécologie médicale
RAVEL Célia Histologie; embryologie et cytogénétique
RIFFAUD Laurent Neurochirurgie
RIOUX-LECLERCQ Nathalie Anatomie et cytologie pathologiques ROBERT-GANGNEUX Florence Parasitologie et mycologie
SAINT-JALMES Hervé Biophysique et médecine nucléaire
SEGUIN Philippe Anesthésiologie-réanimation; médecine d'urgence
SEMANA Gilbert Immunologie
SIPROUDHIS Laurent Gastroentérologie; hépatologie; addictologie
SOMME Dominique Médecine interne; gériatrie et biologie du vieillisement; addictologie
SULPICE Laurent Chirurgie générale
TATTEVIN Pierre Maladies infectieuses; maladies tropicales
THIBAULT Ronan Nutrition
THIBAULT Vincent Bactériologie-virologie; hygiène hospitalière
THOMAZEAU Hervé Chirurgie orthopédique et traumatologique
TORDJMAN Sylvie Pédopsychiatrie; addictologie
VERGER Christian
Professeur des Universités émérite Médecine et santé au travail
VERHOYE Jean-Philippe Chirurgie thoracique et cardiovasculaire
VERIN Marc Neurologie
VIEL Jean-François Epidémiologie, économie de la santé et prévention
VIGNEAU Cécile Néphrologie
VIOLAS Philippe Chirurgie infantile
WATIER Eric Chirurgie plastique, reconstructrice et esthétique; brûlologie
LISTE DES ENSEIGNANTS
MAITRES DE CONFERENCES DES UNIVERSITES – PRACTICIENS HOSPITALIERS
AME-THOMAS Patricia Immunologie
AMIOT Laurence Hématologie; transfusion
BARDOU-JACQUET Edouard Gastroentérologie; hépatologie; addictologie
BEGUE Jean-Marc Physiologie
BOUSSEMART Lise Dermato-vénéréologie
CABILLIC Florian Biologie cellulaire
CAUBET Alain Médecine et santé au travail
DAMERON Olivier Informatique
DE TAYRAC Marie Biochimie et biologie moléculaire
DEGEILH Brigitte Parasitologie et mycologie
DUBOURG Christèle Biochimie et biologie moléculaire
DUGAY Frédéric Histologie; embryologie et cytogénétique
EDELINE Julien Cancérologie; radiothérapie
GALLAND Françoise Endocrinologie, diabète et maladies métaboliques; gynécologie médicale GARLANTEZEC Ronan Epidémiologie, économie de la santé et prévention
GUILLET Benoit Hématologie; transfusion
HAEGELEN Claire Anatomie
JAILLARD Sylvie Histologie; embryologie et cytogénétique
LAVENU Audrey Sciences physico-chimiques et technologies pharmaceutiques LE GALL François Anatomie et cytologie pathologiques
LE RUMEUR Elisabeth Physiologie
MARTINS Raphaël Cardiologie
MASSART Catherine Biochimie et biologie moléculaire
MATHIEU-SANQUER Romain Urologie
MENARD Cédric Immunologie
MENER Eric Médecine générale
MILON Joëlle Anatomie
MOREAU Caroline Biochimie et biologie moléculaire
MOUSSOUNI Fouzia Informatique
MYHIE Didier Médecine générale
PANGAULT Céline Hématologie; transfusion
RENAUT Pierric Médecine générale
RIOU Françoise Epidémiologie, économie de la santé et prévention ROBERT Gabriel Psychiatrie d'adultes; addictologie
ROPARS Mickaël Anatomie
SAULEAU Paul Physiologie
TADIÉ Jean-Marc Réamination; médecine d'urgence
TATTEVIN-FABLET Françoise Médecine générale
TURLIN Bruno Anatomie et cytologie pathologiques
VERDIER Marie-Clémence Pharmacologie fondamentale; pharmacologie clinique; addictologie VINCENT Pascal Bactériologie-virologie; hygiène hospitalière
REMERCIEMENTS
A Monsieur le Professeur Bretagne, je vous prie d’accepter ma sincère et profonde
reconnaissance pour avoir accepté de présider ce travail. Vous nous transmettez avec énergie la passion de la gastro-entérologie et je me considère privilégiée d’avoir pu bénéficier de vos enseignements au cours de mon cursus. Je tacherai de m’en souvenir et de mettre en pratique les nombreux conseils que vous nous avez prodigués.
A Monsieur le Professeur Siproudhis, je vous remercie chaleureusement d’avoir accepté de
juger ce travail. Je vous suis surtout infiniment reconnaissante pour votre soutien bienveillant au long de mon parcours, pour les valeurs d’empathie et de respect que vous nous transmettez et enfin pour la compréhension dont vous avez fait preuve et l’aide que vous m’avez apportée au moment de prendre des décisions importantes.
A Madame le Docteur Verdier, je vous prie d’accepter mes sincères remerciements pour
avoir accepté de juger ce travail, et d’y apporter ainsi un regard peut-être plus fondamental.
A Monsieur le Docteur Bouguen, je te remercie infiniment de m’avoir proposée ce travail et
de m’avoir accompagnée sans relâche à chaque étape de sa réalisation. Merci tout particulièrement pour la passion qui t’anime, ton immense disponibilité ainsi que la patience et la pédagogie dont tu as fait preuve au long de mon apprentissage théorique et pratique.
A tous ceux qui, de près ou de loin, ont contribué à rendre mon parcours parmi vous si riche
A Maman. Il n’y a pas assez de mots pour te dire toute l’admiration que je te porte et toute la
reconnaissance qui est la mienne pour ta présence attentive et affectueuse et ton indéfectible soutien au long de ces années. Tu as créé le doux climat propice à notre épanouissement et nous ne pouvons qu’éternellement t’en remercier.
A Papa et Pascale. Sans vous, rien n’aurait commencé…Je « réalise mon rêve » tandis que
vous vous êtes occupés du « reste » ! Et dans votre regard bienveillant et la constance de vos encouragements, l’on ne pouvait que puiser l’énergie de l’accomplissement personnel.
A ma belle fratrie, mes modèles : Nico, grand frère attentionné, si éloigné et pourtant si
proche, Valou, ma confidente, si éternellement protectrice, PC, mon « Prof » préféré, Doudou le « Magnifique », Mimie ma rêveuse anarchique sans oublier Tutur, brillant de désinvolture. A leurs côtés Maud, Julie, Sophie, Chadi, … à vous aussi Merci.
A la Diaspora Caennaise ! Qui eut prédit de pareilles rencontres ? Myrtille, bouillon
d’humour et d’affection, Adèle, mélange de malice et d’attentions, Tony, la “force tranquille”, dont la répartie me régale, Feufeu mon bobo-socialo-intello préféré, Anne-Cé, ma copine de « bac-à-sable », présente affectueusement et fidèlement depuis toujours et à son cou Walter, aussi farceur que fin d’esprit. Ma joie de vous connaître est indicible et vous savoir à mes côtés au quotidien m’est précieux !
Sans oublier le reste de la troupe, Anaïs, Lulu, Toc Toc, Mathilde, Willou, Féfé, Mathilde, Marco, Haribo, Léa…les souvenirs sont nombreux et l’avenir plein de promesses !
Aux Amitiés plus récentes, Sophie, Ariane, Gothland, Karine, Agathe, Niels, Simon, Agathe,
Pauline, Marine, Justin, Luc, Boubou, Erwan…et à l’espoir de moments riches de convivialité et d’affection.
Une attention toute particulière à celui qui m’a supportée ces derniers mois et su endurer
mes doutes et remises en question. Celui qui par son humour fait du quotidien un Jeu et par sa chaleur de la routine un Bonheur.
Le taux de 6-TGN est-il associé à la rémission
clinique et la cicatrisation muqueuse au cours
des MICI traitées par combothérapie ?
Are 6-Thioguanine Nucleotide Levels
Associated with Clinical Remission and
Mucosal Healing during combination therapy
TABLE DES MATIERES
RESUME………... 13
ABSTRACT……….. 14
INTRODUCTION………. 15
PATIENTS AND METHODS ……… - Study population ………. - Data collection ……… - Outcomes and definitions ………... - Statistical analysis ………... 17 17 17 18 18 RESULTS ……… - Study population and treatments ………. - Clinical steroid-free remission at week 14 ……… - Clinical steroid-free remission at 1 year ………. - Mucosal healing ……….. - Abdominal surgery related to IBD ……….. - Adverse events ……… 19 19 21 22 23 25 28 DISCUSSION ……….. 29 CONCLUSION ……… 32 REFERENCES ………. 33 TABLES OF ILLUSTRATIONS ……… 36
RESUME
Introduction: Le traitement des maladies inflammatoires chroniques de l’intestin (MICI)
modérées à sévères repose sur la combinaison d’immunosuppresseurs et d’anti-TNF. Les 6-thioguanine nucléotides (6-TGN) sont les métabolites actifs des bases puriques. L’objectif de cette étude était d’évaluer l’association entre le taux de 6-TGN et la rémission clinique sans corticoïdes et la cicatrisation muqueuse chez les patients porteurs d’une MICI traitée par combothérapie.
Patients et méthodes : Il s’agissait d’une étude rétrospective monocentrique ayant inclus tous
les patients porteurs d’une MICI, traités par l’association Azathioprine/6-Mercaptopurine et Infliximab/Adalimumab pour lesquels un dosage de 6-TGN était disponible. Les critères de jugement étaient la rémission à 3 et 12 mois, la cicatrisation muqueuse et le recours à la chirurgie.
Résultats : Deux-cent-cinq patients ont été inclus et suivis sur une durée moyenne de 5,9
3,5 ans. Les taux de rémission sans corticoïdes à 3 et 12 mois étaient de 38,5 % et 42 % respectivement sans association significative avec le taux de 6-TGN (p=0,32 et 0,46 respectivement). Cent-soixante-neuf patients (82 %) ont bénéficié d’une coloscopie au cours du suivi parmi lesquels une cicatrisation muqueuse a été objectivée dans 52 % des cas (88/169). Les probabilités cumulées de cicatrisation muqueuse étaient de 14 %, 32 % et 44 % à 1, 3 et 5 ans respectivement. L’obtention d’une cicatrisation muqueuse était corrélée au taux de 6-TGN de façon significative (HR=4,7 (IC 95% 1,1–16,4, p=0,04) pour le seuil de 430 pmol/8.108RBCs. Le taux de chirurgies abdominales en lien avec la MICI était de 24,8 % après un délai médian de 5,1 3,4ans. Les probabilités cumulées de chirurgie étaient de 6 %, 15 % et 22 % à 1, 3 et 5 ans respectivement. Le recours à la chirurgie était corrélé significativement au taux de 6-TGN (HR=2,5, IC 95%1,4–4,5, p=0,004) pour le seuil de 230 pmol/8.108RBCs.
Conclusion : Les résultats de cette étude ne montrent pas d’association significative entre le
taux de 6-TGN et la rémission clinique qu’elle soit à 3 ou 12 mois mais les taux de 6-TGN élevés sont associés à un taux plus élevé de cicatrisation muqueuse et une réduction du recours à la chirurgie au cours du suivi.
ABSTRACT
Background and Study aims: Inflammatory bowel diseases (IBD) are treated with a
combination therapy of immunomodulators and biologics. 6-thioguanine nucleotides (6-TGN) are the active metabolites of thiopurines. The aim of this study was to investigate the association between 6TGN level and clinical steroid-free remission and mucosal healing in patients with IBD treated with combination therapy.
Patients and Methods: A retrospective monocentric study was performed. All confirmed
IBD patients treated with an association of azathioprine/6-mercaptopurine and infliximab/adalimumab and for whom a 6-TGN measurement was available were included. Primary end points were clinical steroid-free remission at 3 and 12 months, mucosal healing and need for surgery.
Results: A total of 205 patients were included and followed during a mean time of 5.9 3.5
years. Clinical steroid-free remission rates at 3 and 12 months were 38.5% and 42% respectively. There was no significant association between 6-TGN level and clinical remission at 3 or 12 months (p=0.32 and 0.46 respectively). A total of 169 patients (82%) had a colonoscopy during the following and 52% of them (88/169) achieved mucosal healing. Cumulative probabilities of mucosal healing were 14%, 32% and 44% at 1, 3 and 5 years respectively. Mucosal healing was significantly correlated with 6-TGN level (HR 4.7, CI 95% 1.1–16.4, p=0.04) for the cut off of 430 pmol/8.108RBCs. A total of 24.8% of patients
underwent an abdominal surgery related to IBD after a mean time of 5,1 3,4 years. Cumulative probabilities of surgery were 6%, 15% and 22% at 1, 3 and 5 years respectively. Abdominal surgery was significantly associated with 6-TGN level (HR=2.5, CI 95% 1.4– 4.5, p=0.004) for the cut off of 230 pmol/8.108RBCs.
Conclusion: This study found no association between 6-TGN levels and clinical remission at
3 and 12 months but suggested that high 6-TGN levels increased the rate of mucosal healing and decreased the need for surgery among IBD patients treated with combination therapy.
Key Words : Inflammatory bowel disease, combination therapy, 6 thioguanine nucleotide, mucosal healing
Abbreviations used : CD, Crohn’s disease - UC, ulcerative colitis – IBD, inflammatory bowel disease – IM, immunomodulator – AZA, azathioprine – MP, 6-mercaptopurine – IFX, infliximab – ADA, adalimumab – anti-TNF, anti tumor necrosis factor alpha - 6-TGN, 6 thioguanine nucleotides –6MMP, 6 méthylmercaptopurine - RBCs, red blood cells – HBI, Harvey Bradshaw Index – CRP, C reactive protein- ROC, Receiver operating characteristic- MCV, Mean corpuscular volume- TPMT, thiopurines S-methyl-transferase
INTRODUCTION
Inflammatory bowel diseases (IBD) namely Crohn’s disease (CD) and ulcerative colitis (UC) are chronic inflammatory conditions. The treatment of IBD includes immunomodulators (IM) and biologics. Two of the most widely used immunomodulators are 6-mercaptopurine (MP) and its pro-drug, azathioprine (AZA).
Both azathioprine and 6-mercaptopurine are converted following a multi-step enzymatic process to active metabolites, namely 6-thioguanine nucleotides (6-TGN). 6-TGN act as antagonists to the endogenous purines, which are essential components of DNA and RNA. A 6TGN level greater than 230 pmol/8.108 red blood cells (RBCs) has been associated with clinical remission in patients with CD, that was confirmed in several meta-analysis1,2.
Several studies showed the combination therapy with infliximab (IFX), an anti-tumor necrosis factor agent (anti-TNF), and thiopurines as the most effective therapy for both CD and UC. The SONIC trial and the SUCCESS trial compared IFX monotherapy, AZA monotherapy and combination therapy of the two drugs in 508 adults with moderate-to-severe CD and 239 adults with moderate-to-severe UC, respectively. Patients treated with combination therapy were significantly more likely to be in clinical steroid-free remission at week 26 and 50 than those in monotherapy for both trials3,4. The additive efficacy of each drug and pharmacokinetic interaction (reduction of immunogenicity with a resultant increase in serum anti-TNF levels) might explain the greater efficacy of the combination therapy5,6. Unfortunately, combination therapy was also associated with higher risk of infection and T-cell lymphoma related to thiopurines use7–9. Some studies among non IBD-patients observed a relationship between risk of malignancy and thiopurines dose or metabolite level10,11. These data raised the question of the optimal use of thiopurines during combination therapy to minimize toxicity without decreasing the efficacy and the pharmacokinetic profile on serum anti-TNF levels.
To date, most studies on combination therapy used full weight-based thiopurines doses (AZA-2.0-2.5 mg/kg per day, MP-1.0-1.5mg/kg per day) with or without further dose optimization based on 6TGN levels3,12
Retrospective studies found no correlation between IM doses or 6-TGN levels and anti-TNF levels suggesting that lower IM doses in combination therapy may be equally effective 13,14.
Recently, a prospective study observed a correlation between 6TGN levels and infliximab trough concentrations. 6-TGN level of 125 pmol/8.108 RBCs was sufficient to obtain higher
IFX trough concentration and avoid the onset of ATIs. Authors suggested that usual therapeutic levels of 6-TGN did not confer additional benefit on IFX trough levels 15. Taken
together, these studies raised the fact that low 6TGN levels may be sufficient to reduce immunogenicity and to maintain therapeutic infliximab trough levels. However, the postulate of these studies was based on an efficacy of the combination therapy mainly driven by the anti-TNF without clinical impact of purine analogues. Clinical endpoints such as clinical remission or mucosal healing were not assessed.
The aim of this study was to investigate whether lower 6TGN levels are equally effective to achieve clinical endpoints (steroid-free remission, mucosal healing, and need for surgery) in patients with IBD treated with combination therapy
PATIENTS AND METHODS
Study Population
All dosages of 6TGN between January 2004 and April 2014 were retrieved from the database of the pharmacology unit. The database was then compared to the records of all patients treated with anti-TNF (IFX and ADA). Charts of selected patients were then reviewed. All patients with a documented and confirmed diagnosis of CD or UC treated with a combination therapy with IFX/ADA and AZA/MP and for whom a 6TGN level was available were included.
Data collection
The following variables were considered: demographics, size, weight, Body mass index (BMI), smoking status, date of IBD diagnosis, IBD phenotype and extent of disease according to the Montreal classification16, prior treatments including steroids, mesalasine, immunomodulators and anti-TNF monotherapy and prior abdominal surgeries. The following data were collected at the initiation of combination therapy, week 14 and 1 year: date of first administration of anti-TNF, IBD phenotype and extent of disease according to the Montreal classification, severity of the disease according to the Harvey Bradshaw index (HBI)17 for CD patients and the Mayo score18 for UC patients, biological data including complete blood count and C reactive protein (CRP) concentration, concomitant medications including steroids and mesalasine.
In case of repeated 6-TGN and 6-MMP levels measurements, the closest dosage from the initiation of combination therapy without a dose change of purine analogue during the follow-up was selected for further analysis. 6TGN and 6MMP levels were assessed 4 to 6 hours following pills intake. A complete blood count was systematically associated.
The combination therapy modalities were detailed: anti-TNF treatment modalities (induction alone versus induction plus maintenance therapy), potential optimization and treatment cessation. Adverse events were defined as any clinical or biological event following combination therapy initiation that could be relied on treatment.
The results of ileo-colonoscopies during the follow-up were reported as well as the occurrence of any abdominal surgery.
Outcomes and definitions
Clinical steroid-free remission was defined for CD as follows: a HBI<4 and CRP concentration < 5 mg/L without any steroid treatment and surgery. Clinical steroid-free remission was defined for UC as follows: Partial Mayo score < 2 and CRP concentration < 5 mg/L without any steroid treatment and surgery. Clinical steroid-free remission was checked at week 14 and 1 year.
Mucosal healing was defined by the absence of ulceration for CD and a Mayo score of 0 or 1 for UC19–21. All abdominal surgeries related to IBD following initiation of combination therapy were considered.
Statistical analysis
Quantitative variables were described as means and standard deviations (SD). Categorical variables were presented as counts and percent of the cohort.
To look for baseline factors that could predict responses at week 14 and 1 year, univariate analyses were performed using Student’s t test for quantitative variables and a chi-square test for qualitative variables. All items obtained by univariate analysis with a p-value below 0.2 were integrated into a logistic regression model for multivariate analysis.
Survival analysis was used for mucosal healing and surgery. The cumulative probabilities of survival without event were estimated by using the Kaplan-Meier method. The time to mucosal healing and surgery were considered to begin at the date of combination therapy initiation and end at the date of the event. Variables associated with each event (p<0.05) by univariate analysis (log-rank) were integrated into a cox model for multivariate analysis. Receiver operating characteristic (ROC) analysis was performed to determine the optimal cut-off for 6-TGN level that predicts outcomes. We also used the cut-cut-off of 230 pmol/8.108 RBCs that was previously associated with clinical outcomes in patients treated with thiopurines on monotherapy1,2.
A p-value of <0.05 was considered statistically significant for analysis. All analyses were performed using JMP Pro 10.0.2 software (SAS Institute Inc.,Cary,NC).
RESULTS
Study population and treatments
A total of 205 patients (158 CD and 47 UC) were included and followed over a mean time period of 5.93.5 years. Baseline characteristics of patients are depicted in Table 1.
A total of 179 patients (87%) received a combination therapy with infliximab and 26 (13%) with adalimumab. Among them, 162 patients (79%) were already treated by azathioprine monotherapy, 10 of 205 (5%) by anti-TNF monotherapy and 33 patients (16%) started simultaneously both drugs.
Regarding anti-TNF treatment modalities, 25% of patients (52/205) received only induction therapy whereas 75% of them (153/205) received induction plus maintenance therapy.
Anti-TNF treatment was still ongoing for 54 of 205 patients (26%) at last news of which optimization was performed in 35 cases. After a mean time period of 21458 weeks, 149 of 205 patients (73%) discontinued anti-TNF treatment of which 57 before one year of combination therapy. Reasons for discontinuation were the followings: 47 patients achieved sustained clinical benefits, 42 patients only received an induction therapy, 32 patients were primary or secondary non-responders, 12 patients experienced adverse events, 5 patients had a surgery, 5 patients discontinued treatment for pregnancy and 6 patients for personal convenience.
Regarding IM therapy, 68 of 205 patients (33%) were still treated at last news. Patients discontinued IM for the following reasons: 58 patients achieved clinical benefits, 26 patients were primary or secondary non-responders, 22 patients experienced adverse events, 16 patients discontinued treatment for personal convenience, 11 patients had a surgery and 2 patients discontinued treatment for pregnancy.
The mean duration of combination therapy was 16.71.6 months. All patients were still on combination therapy at week 14 and 131 of 205 patients (64%) were still on combination therapy at one year. A total of 19 patients (9%) continued combination therapy until the end of the follow-up.
6-TGN levels were available for all patients and 6-MMP levels for 97.6% (200/205) of patients.
Mean 6TGN level was 339 205 pmol/8.108 RBCs. Quartile analysis revealed that 25% of patients had 6-TGN level below 235, 25% between 235 and 293, 25% between 293 and 388 and 25% above 388 pmol/8.108RBCs.
Mean 6MMP level was 1610 2094 pmol/8. 108 RBCs. Quartile analysis revealed that 25% of patients have 6-MMP level below 426.5, 25% between 426.5 and 929, 25% between 929 and 1820 and 25% above 1820 pmol/8. 108 RBCs.
6-TGN/6-MMP measurement was performed after a mean time of 7.613.8 months following initiation of combination therapy.
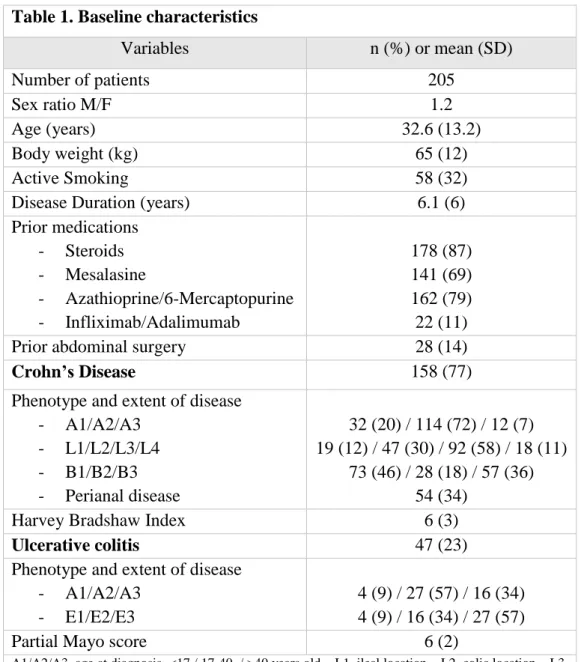
Table 1. Baseline characteristics
Variables n (%) or mean (SD) Number of patients 205 Sex ratio M/F 1.2 Age (years) 32.6 (13.2) Body weight (kg) 65 (12) Active Smoking 58 (32)
Disease Duration (years) 6.1 (6)
Prior medications - Steroids - Mesalasine - Azathioprine/6-Mercaptopurine - Infliximab/Adalimumab 178 (87) 141 (69) 162 (79) 22 (11)
Prior abdominal surgery 28 (14)
Crohn’s Disease 158 (77)
Phenotype and extent of disease - A1/A2/A3 - L1/L2/L3/L4 - B1/B2/B3 - Perianal disease 32 (20) / 114 (72) / 12 (7) 19 (12) / 47 (30) / 92 (58) / 18 (11) 73 (46) / 28 (18) / 57 (36) 54 (34)
Harvey Bradshaw Index 6 (3)
Ulcerative colitis 47 (23)
Phenotype and extent of disease - A1/A2/A3
- E1/E2/E3
4 (9) / 27 (57) / 16 (34) 4 (9) / 16 (34) / 27 (57)
Partial Mayo score 6 (2)
A1/A2/A3, age at diagnosis <17 / 17-40 / >40 years old – L1, ileal location – L2, colic location – L3, ileocolic location – L4, isolated upper disease location – B1, non stricturing non penetrating behavior – B2 stricturing behavior – B3, penetrating behavior – E1, proctitis – E2 left colitis – E3, pancolitis
Clinical steroid-free remission at week 14
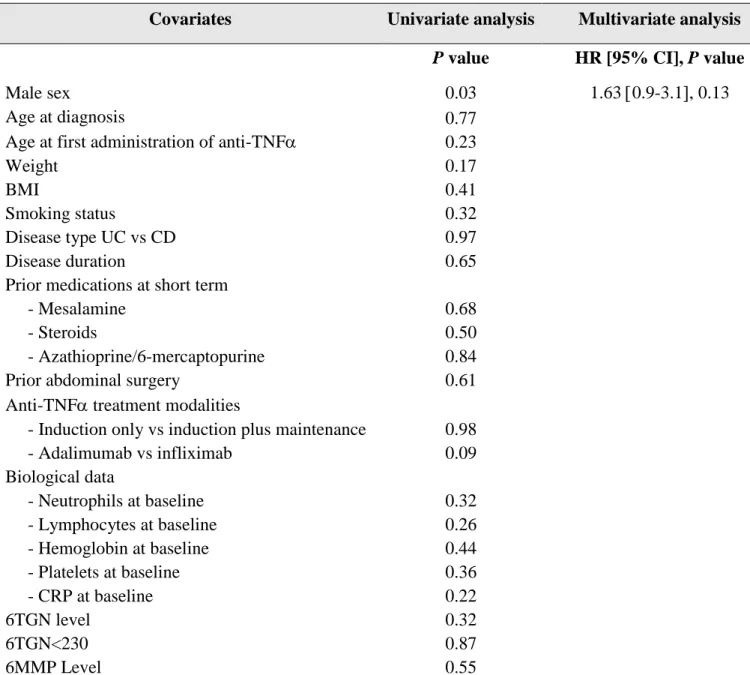
A total of 38.5% of patients (79/205) were on steroid-free remission at week 14.
By multivariate analysis, no baseline characteristic was associated with clinical steroid-free remission at week 14 (Table 2).
Neither 6TGN level nor 6-TGN cut-off of 230 pmol/8.108 RBCs were associated with clinical steroid-free remission at week 14.
Table 2. Factors associated with clinical steroid-free remission at week 14
Covariates Univariate analysis Multivariate analysis
P value HR [95% CI], P value
Male sex 0.03 1.63 0.9-3.1, 0.13
Age at diagnosis
Age at first administration of anti-TNF
0.77 0.23 Weight BMI Smoking status 0.17 0.41 0.32 Disease type UC vs CD 0.97 Disease duration 0.65
Prior medications at short term
- Mesalamine 0.68
- Steroids 0.50
- Azathioprine/6-mercaptopurine 0.84
Prior abdominal surgery 0.61
Anti-TNF treatment modalities
- Induction only vs induction plus maintenance 0.98
- Adalimumab vs infliximab 0.09 Biological data - Neutrophils at baseline 0.32 - Lymphocytes at baseline 0.26 - Hemoglobin at baseline 0.44 - Platelets at baseline 0.36 - CRP at baseline 0.22 6TGN level 6TGN<230 0.32 0.87 6MMP Level 0.55
Anti-TNF, anti tumor necrosis factor alpha – BMI, Body Mass Index – UC, ulcerative colitis – CD, Crohn’s disease – 6TGN, 6 Thioguanine Nucleotide – 6MMP, 6 Methylmercaptopurine.
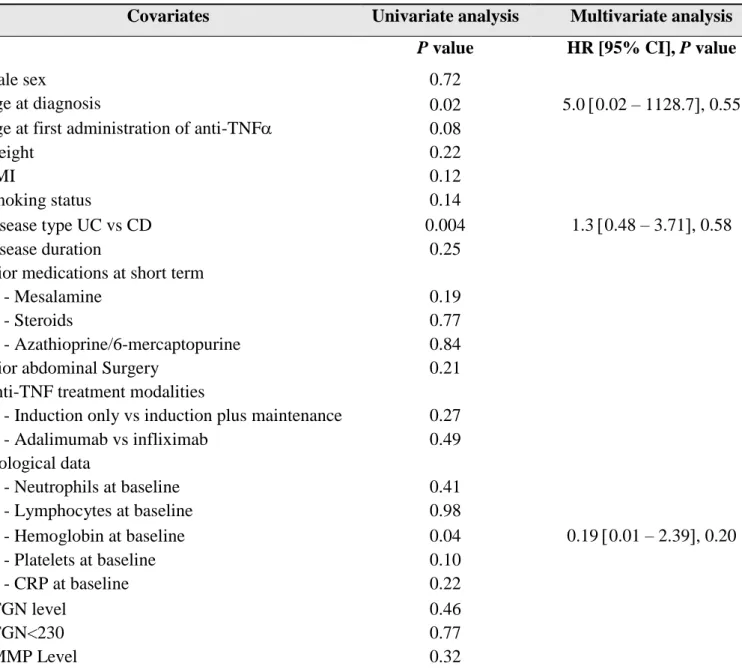
Clinical steroid-free remission at 1 year
A total of 87 patients (42%) were on clinical steroid-free remission at 1 year.
Baseline factors associated with clinical steroid-free remission at one year by univariate analysis were the age at diagnosis (p=0.02), disease type (p=0.004) and hemoglobin concentration at baseline (p=0.04). None of these factors was associated with clinical-steroid free remission at 1 year by multivariate analysis (Table 3)
Neither 6TGN level nor 6-TGN cut-off of 230 pmol/8.108 RBCs were associated with clinical steroid-free remission at one year.
Table 3. Factors associated with clinical steroid-free remission at one year
Covariates Univariate analysis Multivariate analysis
P value HR [95% CI], P value
Male sex 0.72
Age at diagnosis
Age at first administration of anti-TNF
0.02 0.08 5.0 0.02 – 1128.7, 0.55 Weight BMI Smoking status 0.22 0.12 0.14 Disease type UC vs CD 0.004 1.3 0.48 – 3.71, 0.58 Disease duration 0.25
Prior medications at short term
- Mesalamine 0.19
- Steroids 0.77
- Azathioprine/6-mercaptopurine 0.84
Prior abdominal Surgery 0.21
Anti-TNF treatment modalities
- Induction only vs induction plus maintenance 0.27
- Adalimumab vs infliximab 0.49 Biological data - Neutrophils at baseline 0.41 - Lymphocytes at baseline 0.98 - Hemoglobin at baseline 0.04 0.19 0.01 – 2.39, 0.20 - Platelets at baseline 0.10 - CRP at baseline 0.22 6TGN level 6TGN<230 0.46 0.77 6MMP Level 0.32
Anti-TNF, anti tumor necrosis factor alpha – BMI, Body Mass Index – UC, ulcerative colitis – CD, Crohn’s disease – 6TGN, 6 Thioguanine Nucleotide – 6MMP, 6 Methylmercaptopurine
Mucosal healing
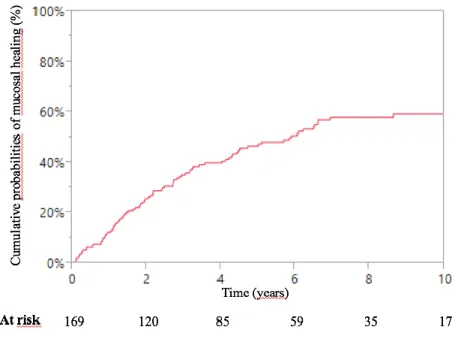
A total of 169 patients (82%) had at least one ileocolonoscopy after starting combination therapy.
The mean time between initiation of combination therapy and the first endoscopic procedure was 28.9 24.7 months.
There were 52% of patients evaluated (88/169) who achieved mucosal healing.
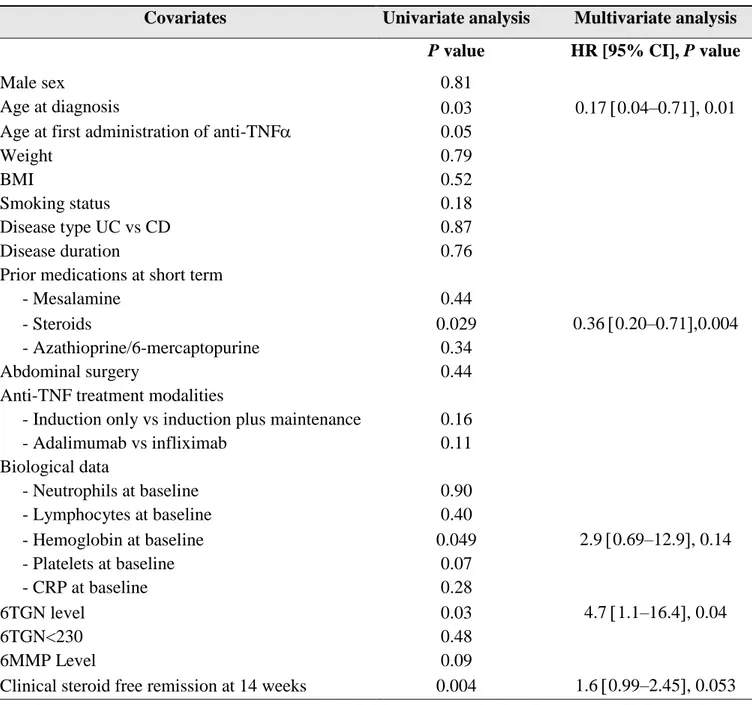
The cumulative probabilities of achieving mucosal healing after starting combination therapy were 14%, 32% and 44% at 1, 3 and 5 years respectively (Figure 1).
Figure 1 – Kaplan Meier curve of cumulative probabilities of mucosal healing following combination therapy for inflammatory bowel disease
Baseline factors significantly associated with mucosal healing by univariate analysis were the age at diagnosis (p=0.03), prior steroid treatment (p=0.029), hemoglobin concentration (p=0.049), 6-TGN level (p-0.03), clinical steroid-free remission at 14 weeks (p=0.004) (Table
4).
The optimal cut-off value for 6-TGN level obtained by ROC curve analysis was 430 pmol/8.108 RBCs.
By multivariate analysis, a 6-TGN level above 430 pmol/8.108 RBCs was significantly and independently associated with mucosal healing with a hazard ratio of 4.7 (95% CI 1.1–16.4, p=0.04) (Table 4 and Figure 2).
Table 4. Factors associated with mucosal healing
Covariates Univariate analysis Multivariate analysis
P value HR [95% CI], P value
Male sex 0.81
Age at diagnosis
Age at first administration of anti-TNF
0.03 0.05 0.17 0.04–0.71, 0.01 Weight BMI Smoking status 0.79 0.52 0.18 Disease type UC vs CD 0.87 Disease duration 0.76
Prior medications at short term
- Mesalamine 0.44
- Steroids 0.029 0.36 0.20–0.71,0.004
- Azathioprine/6-mercaptopurine 0.34
Abdominal surgery 0.44
Anti-TNF treatment modalities
- Induction only vs induction plus maintenance 0.16
- Adalimumab vs infliximab 0.11 Biological data - Neutrophils at baseline 0.90 - Lymphocytes at baseline 0.40 - Hemoglobin at baseline 0.049 2.9 0.69–12.9, 0.14 - Platelets at baseline 0.07 - CRP at baseline 0.28 6TGN level 6TGN<230 0.03 0.48 4.7 1.1–16.4, 0.04 6MMP Level 0.09
Clinical steroid free remission at 14 weeks 0.004 1.6 0.99–2.45, 0.053
Anti-TNF, anti tumor necrosis factor alpha – BMI, Body Mass Index – UC, ulcerative colitis – CD, Crohn’s disease – 6TGN, 6 Thioguanine Nucleotide – 6MMP, 6 Methylmercaptopurine
Figure 2 – Kaplan Meier curve of cumulative probabilities of mucosal healing during combination therapy for inflammatory bowel disease according to 6-TGN level
Abdominal Surgery related to IBD
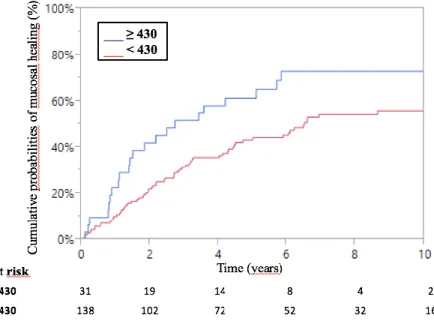
After a mean follow-up of 5.13.4 years, 24.8% of patients (51/205) underwent an abdominal surgery, including 35 CD patients and 16 UC patients. Types of surgery were ileal surgeries for 24 patients (47%) and colonic surgeries for 27 patients (53%).
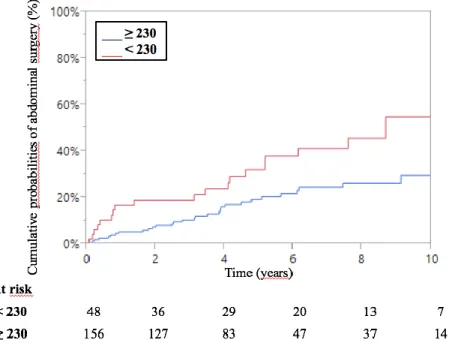
The cumulative probabilities of abdominal surgery were 6%, 15% and 22% at 1, 3 and 5 years respectively (Figure 3).
Figure 3 – Kaplan Meier curve of cumulative probabilities of abdominal surgery related to IBD following combination therapy for inflammatory bowel disease.
Baseline factors significantly associated with abdominal surgery by univariate analysis were: disease type (p = 0.04), 6-TGN cut-off value of 230 pmol/8.108 RBCs (p=0.017) and clinical
steroid-free remission at 14 weeks (p=0.01) (Table 5).
By multivariate analysis, the 6-TGN level < 230 pmol/8.108 RBCs was significantly and independently associated with abdominal surgery with a hazard ratio of 2.5 (95%CI 1.4–4.5, p=0.004) (Table 5 and Figure 4)
Table 5. Factors associated with abdominal surgery related to IBD
Covariates Univariate analysis Multivariate analysis
P value HR [95% CI], P value
Male sex 0.53
Age at diagnosis Age at first IFX
0.32 0.6 Weight BMI Smoking status 0.21 0.18 0.28 Disease type UC vs CD 0.04 2.41.3–4.5, 0.01 Disease duration 0.35
Prior medications at short term
- Mesalamine 0.19
- Steroids 0.08
- Azathioprine/6-mercaptopurine 0.88
Surgery 0.94
Anti-TNF treatment modalities
- Induction only vs induction plus maintenance 0.19
- Adalimumab vs infliximab 0.71 Biological data - Neutrophils at baseline 0.77 - Lymphocytes at baseline 0.87 - Hemoglobin at baseline 0.30 - Platelets at baseline 0.57 - CRP at baseline 0.78 6TGN level 6TGN<230 0.28 0.017 2.51.4–4.5, 0.004 6MMP Level 0.58
Clinical steroid free remission at 14 weeks 0.01 0.4 0.16–0.70, 0.002
Anti-TNF, anti tumor necrosis factor – BMI, Body Mass Index – UC, ulcerative colitis – CD, Crohn’s disease – 6TGN, 6 Thioguanine Nucleotide – 6MMP, 6 Methylmercaptopurine
Figure 4 – Kaplan Meier curve of cumulative probabilities of surgery following combination therapy for inflammatory bowel disease according to 6-TGN level
Adverse events
A total of 77 adverse events (37.6%) following combination therapy were reported from the chart of the 205 included patients.
Lymphocytopenia concerned 41 patients (20%) and lead to decrease IM posology in 2 cases. Infectious complications concerned 14 patients (6.8%) and included one tuberculosis, one chicken pox, three herpes zoster, one colitis due to cytomegalovirus and eight minor viral infections.
Other side effects were immune-allergic reaction (immediate hypersensitivity reaction) in 15 patients, psoriasis in one patient, pancreatitis in one patient, thrombo-embolic event in two patients, depression in one patient, suspicion of multiple sclerosis in one patient and kidney cancer in one patient.
Mean 6-TGN level among patients who developed adverse events was 340206 pmol/8.108 RBCs.
DISCUSSION
6-TGN level was associated with clinical remission only in IBD patients treated with thiopurines monotherapy 1, and few data are actually available during combination therapy. Some studies suggested improved outcomes of IBD patients treated with combination therapy linked to a decrease of immunogenicity 5,6. For such purpose, lower 6-TGN levels might be sufficient to reduce this immunogenicity 15, but association between 6-TGN level and clinical outcomes remained to be determined to assess the proper effect of purine analogues.
In the present study, 6-TGN levels were not associated with clinical steroid-free remission at week 14 and 1 year. However, positive links between higher 6-TGN levels and mucosal healing as well as abdominal surgery were observed in patients with IBD treated with combination therapy. Such findings support the hypothesis of the own effect of IM for improving outcomes of IBD patients treated with combination therapy.
Based on the fact that combination therapy increases the risk of infections and lymphoma 7–9,
some investigators proposed IM discontinuation when remission was obtained. Following such strategy half patients relapse after thiopurines discontinuation underlining this possible effect of thiopurines 12,22. The Yarur study tested an alternative strategy consisting in
thiopurines dose reduction in case of remission. Lower 6-TGN levels (125 pmol/8.108 RBCs) as compared to usual therapeutic levels (230 pmol/8.108RBCs) were sufficient to maintain therapeutic IFX trough levels meaning that low 6-TGN could be sufficient to maintain pharmacokinetic effect with a reduced toxicity but no clinical endpoint was assessed. Accordingly, authors showed that a level of IFX of 8.3 µg/ml best predicted mucosal healing. That said, similar findings to the present study were observed regarding purine analogues :a 6-TGN level of 223 pmol/8.108 RBCs was associated with mucosal healing 15.
In a post-hoc analysis from SONIC trial, ΔMCV (mean corpuscular volume), a surrogate for 6-TGN concentrations, was associated with mucosal healing among patients treated with combination therapy. A significant correlation between a ΔMCV above 7, which is known to be correlated with a mean 6-TGN concentration >255 pmol/8.108 RBCs, and mucosal healing were observed. These results are in line with our study by showing that thiopurines themselves lead to increase efficacy according to the endpoint of mucosal healing 23.
Similarly, in the CONCERTO trial, rheumatoid arthritis patients were randomized to open – label adalmumab 40mg every other weeks and blinded oral methotrexate at dose of 2.5, 5, 10 or 20mg weekly. ADA serum concentrations increased and anti-adalimumab antibody formation decreased with increasing methotrexate dose. Clinical outcomes were also
improved with dose escalation in favor of a dose-dependent effect of methotrexate, an immunomodulator such as AZA/MP, in combination to anti-TNF therapy24.
This study brings new data about the association between mucosal healing and 6-TGN concentrations in IBD treated with combination therapy. However, several limitations have to be mentioned in addition to the retrospective study design. Clinical remission remains a subjective criteria of evaluation regarding the known discrepancy between symptoms and objective markers of gut inflammation25,26. The correlation between 6-TGN level and mucosal healing but not with clinical remission may be explained by this discrepancy, particularly during Crohn’s disease. In a post-hoc analysis of the SONIC trial, Peyrin-Biroulet et al showed that broadly half patients in clinical remission have endoscopic features of inflammation and conversely, half patients that have achieved mucosal healing remain symptomatic according to the Crohn’s disease activity index27. It can explain, at least in part, the absence of association between clinical remission and 6-TGN levels in our study. Assessment of mucosal healing was retrieved from endoscopy reports without the use of validated scoring system. Nevertheless, the absence of endoscopic ulceration during CD is actually used for the definition of mucosal healing 21. Both clinical remission and mucosal healing may have been under or overestimated.
Regarding 6-TGN measurement, limitations imply the time between initiation of combination therapy and measurement of 6-TGN and 6-MMP concentrations since concentrations may vary with combination therapy 28. Most of patients were tested within the year of the initiation
of the combination therapy and thiopurines dosage was decreased in few patients in case of side effects (n=2). Inter individual bioavailability variations of thiopurines are mainly related to the thiopurines S-methyl-transferase (TPMT) activity that is presumed to remain stable overtime on combination therapy. Confounding variables that may have affected the bioavailability of thiopurines in the present study include mucosal healing itself ( impact on absorption), patient adherence and potential differences between generic and brand name AZA29. Regarding 6-TGN threshold associated with outcomes, the cut-off of 230 pmol/8.108 RBCs associated with the onset of surgery is in line with cut-off associated with clinical remission in previous studies on thiopurines monotherapy. Nevertheless, the cut-off of 430 pmol/8.108 RBCs associated with mucosal healing is quite unusual. In the meta-analysis, cut-off used ranged from 230 to 260 pmol/8.108 RBCs and mean 6-TGN value among patients in clinical remission ranged from 166 to 325 pmol/8.108 RBCs. But mucosal healing were not assessed1,2. It can be presumed that higher 6-TGN levels are needed to achieve mucosal healing.
Regarding adverse events, only 12 patients discontinued anti-TNF and 22 patients IM because of side effects. There were only six major infections and one case of kidney cancer but no case of lymphoma or skin cancer. That said, combination therapy with full-weight dose of thiopurines seems acceptable from a tolerance point of view.
CONCLUSION
Thiopurines and TNF antagonists therapeutic drug monitoring might be used to improve disease outcomes in IBD patients. The present study found no association between 6-TGN levels and clinical remission at week 14 and 1 year but suggested that higher levels of 6-TGN led to increase mucosal healing and decrease the need for surgery among IBD patients treated with combination therapy. These results favour the use of full thiopurines dose during combination therapy related.
REFERENCES
1. Moreau AC, Paul S, Del Tedesco E, et al. Association Between 6-Thioguanine Nucleotides Levels and Clinical Remission in Inflammatory Disease: A Meta-analysis.
Inflamm Bowel Dis 2014; 20: 464–471.
2. Osterman MT, Kundu R, Lichtenstein GR, et al. Association of 6-Thioguanine Nucleotide Levels and Inflammatory Bowel Disease Activity: A Meta-Analysis.
Gastroenterology 2006; 130: 1047–1053.
3. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. N Engl J Med 2010; 362: 1383–1395.
4. Panaccione R, Ghosh S, Middleton S, et al. Combination Therapy With Infliximab and Azathioprine Is Superior to Monotherapy With Either Agent in Ulcerative Colitis.
Gastroenterology 2014; 146: 392–400.e3.
5. Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007; 56: 1226–1231.
6. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003; 348: 601–608.
7. Siegel CA, Marden SM, Persing SM, et al. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am
Gastroenterol Assoc 2009; 7: 874–881.
8. Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of Lymphoma in Patients With Inflammatory Bowel Disease Treated With Azathioprine and 6-Mercaptopurine: A Meta-analysis. Clin Gastroenterol Hepatol 2015; 13: 847–858.e4.
9. Toruner M, Loftus EV, Harmsen WS, et al. Risk Factors for Opportunistic Infections in Patients With Inflammatory Bowel Disease. Gastroenterology 2008; 134: 929–936.
10. Lennard L, Thomas S, Harrington CI, et al. Skin cancer in renal transplant recipients is associated with increased concentrations of 6-thioguanine nucleotide in red blood cells. Br J
Dermatol 1985; 113: 723–729.
11. Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs 2007; 67: 1167–1198.
12. Van Assche G, Magdelaine-Beuzelin C, D’Haens G, et al. Withdrawal of
immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology 2008; 134: 1861–1868.
13. van Schaik T, Maljaars JPW, Roopram RK, et al. Influence of Combination Therapy with Immune Modulators on Anti-TNF Trough Levels and Antibodies in Patients with IBD:
Inflamm Bowel Dis 2014; 20: 2292–2298.
14. Cahill J, Zadvornova Y, Naik AS, et al. Tu1282 Azothioprine or 6-Mercaptopurnine Dose Does Not Effect Serum Infliximab Level or Rate of Antibody to Infliximab Formation.
Gastroenterology 2015; 148: S–847.
15. Yarur AJ, Kubiliun MJ, Czul F, et al. Concentrations of 6-Thioguanine Nucleotide Correlate With Trough Levels of Infliximab in Patients With Inflammatory Bowel Disease on Combination Therapy. Clin Gastroenterol Hepatol 2015; 13: 1118–1124.e3.
16. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol J Can
Gastroenterol 2005; 19 Suppl A: 5A–36A.
17. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet Lond
Engl 1980; 1: 514.
18. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated Oral 5-Aminosalicylic Acid Therapy for Mildly to Moderately Active Ulcerative Colitis. N Engl J Med 1987; 317: 1625– 1629.
19. Mazzuoli S, Guglielmi FW, Antonelli E, et al. Definition and evaluation of mucosal healing in clinical practice. Dig Liver Dis 2013; 45: 969–977.
20. D’Haens G, Sandborn WJ, Feagan BG, et al. A Review of Activity Indices and
Efficacy End Points for Clinical Trials of Medical Therapy in Adults With Ulcerative Colitis.
Gastroenterology 2007; 132: 763–786.
21. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target.
Am J Gastroenterol 2015; 110: 1324–1338.
22. Oussalah A, Chevaux J-B, Fay R, et al. Predictors of Infliximab Failure After Azathioprine Withdrawal in Crohn’s Disease Treated With Combination Therapy. Am J
Gastroenterol 2010; 105: 1142–1149.
23. Bouguen G, Sninsky C, Tang KL, et al. Change in Erythrocyte Mean Corpuscular Volume During Combination Therapy with Azathioprine and Infliximab Is Associated with Mucosal Healing: A Post hoc Analysis from SONIC. Inflamm Bowel Dis 2015; 21: 606–614. 24. Burmester G-R, Kivitz AJ, Kupper H, et al. Efficacy and safety of ascending
methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann
25. Cellier C, Sahmoud T, Froguel E, et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut 1994; 35: 231–235.
26. Modigliani R. Clinical, biological, and endoscopic picture of attacks of Crohn’s disease. Evolution on prednisolone. Groupe d’Etude Therapeutique des Affections Inflammatoires Digestives. Gastroenterology 1990; 98: 811–818.
27. Peyrin-Biroulet L, Reinisch W, Colombel J-F, et al. Clinical disease activity,
C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 2014; 63: 88–95.
28. Roblin X, Serre-Debeauvais F, Phelip J-M, et al. Drug interaction between infliximab and azathioprine in patients with Crohn’s disease. Aliment Pharmacol Ther 2003; 18: 917– 925.
29. Cuffari C, Theoret Y, Latour S, et al. 6-Mercaptopurine metabolism in Crohn’s disease: correlation with efficacy and toxicity. Gut 1996; 39: 401–406.
TABLE OF ILLUSTRATIONS
Table 1. Baseline characteristics ………20 Table 2. Factors associated with clinical steroid-free remission at week 14………… 21
Table 3. Factors associated with clinical steroid-free remission at one year ………...22 Table 4. Factors associated with mucosal healing ………. 24 Table 5. Factors associated with abdominal surgery related to IBD ……….27
Figure 1 – Kaplan Meier curve of cumulative probabilities of mucosal healing following combination therapy for inflammatory bowel disease………23
Figure 2 – Kaplan Meier curve of cumulative probabilities of mucosal healing during combination therapy for inflammatory bowel disease according to 6-TGN level ……...25
Figure 3 – Kaplan Meier curve of cumulative probabilities of abdominal surgery related to IBD following combination therapy for inflammatory bowel disease………...26
Figure 4 – Kaplan Meier curve of cumulative probabilities of surgery related to IBD following combination therapy for inflammatory bowel disease according to 6-TGN level………...28
VIAUD, Juliette - Le taux de 6-TGN est-il associé à la rémission clinique et la cicatrisation muqueuse au cours des MICI traitées par combothérapie?
36 feuilles, 4 figures, 5 tableaux, 30 cm.- Thèse : (Médecine) ; Rennes 1; 2016 ; N°
Introduction: L’objectif de cette étude était d’évaluer l’association entre le taux de 6-TGN et la rémission clinique sans corticoïdes et la
cicatrisation muqueuse chez les patients porteurs d’une MICI traitée par combothérapie.
Patients et méthodes : Il s’agissait d’une étude rétrospective monocentrique ayant inclus tous les patients porteurs d’une MICI, traités par
l’association Azathioprine/6-Mercaptopurine et Infliximab/Adalimumab pour lesquels un dosage de 6-TGN était disponible. Les critères de jugement étaient la rémission à 3 et 12 mois, la cicatrisation muqueuse et le recours à la chirurgie.
Résultats : Deux-cent-cinq patients ont été inclus et suivis sur une durée moyenne de 5,9 3,5 ans. Les taux de rémission sans corticoïdes à 3
et 12 mois étaient de 38,5 % et 42 % respectivement sans association significative avec le taux de 6-TGN (p=0,32 et 0,46 respectivement). Cent-soixante-neuf patients (82 %) ont bénéficié d’une coloscopie au cours du suivi parmi lesquels une cicatrisation muqueuse a été objectivée dans 52 % des cas (88/169). Les probabilités cumulées de cicatrisation muqueuse étaient de 14 %, 32 % et 44 % à 1, 3 et 5 ans respectivement. L’obtention d’une cicatrisation muqueuse était corrélée au taux de 6-TGN de façon significative (HR=4,7 (IC 95% 1,1–16,4, p=0,04) pour le seuil de 430 pmol/8.108RBCs. Le taux de chirurgies abdominales en lien avec la MICI était de 24,8 % après un délai médian de
5,1 3,4ans. Les probabilités cumulées de chirurgie étaient de 6 %, 15 % et 22 % à 1, 3 et 5 ans respectivement. Le recours à la chirurgie était corrélé significativement au taux de 6-TGN (HR=2,5, IC 95%1,4–4,5, p=0,004) pour le seuil de 230 pmol/8.108RBCs.
Conclusion : Les résultats de cette étude ne montrent pas d’association significative entre le taux de 6-TGN et la rémission clinique qu’elle
soit à 3 ou 12 mois mais les taux de 6-TGN élevés sont associés à un taux plus élevé de cicatrisation muqueuse et une réduction du recours à la chirurgie au cours du suivi.
Background and Study aims: The aim of this study was to investigate the association between 6TGN level and clinical steroid-free remission
and mucosal healing in patients with IBD treated with combination therapy.
Patients and Methods: A retrospective monocentric study was performed. All confirmed IBD patients treated with an association of
azathioprine/6-mercaptopurine and infliximab/adalimumab and for whom a 6-TGN measurement was available were included. Primary end points were clinical steroid-free remission at 3 and 12 months, mucosal healing and need for surgery.
Results: A total of 205 patients were included and followed during a mean time of 5.9 3.5 years. Clinical steroid-free remission rates at 3
and 12 months were 38.5% and 42% respectively. There was no significant association between 6-TGN level and clinical remission at 3 or 12 months (p=0.32 and 0.46 respectively). A total of 169 patients (82%) had a colonoscopy during the following and 52% of them (88/169) achieved mucosal healing. Cumulative probabilities of mucosal healing were 14%, 32% and 44% at 1, 3 and 5 years respectively. Mucosal healing was significantly correlated with 6-TGN level (HR 4.7, CI 95% 1.1–16.4, p=0.04) for the cut off of 430 pmol/8.108RBCs. A total of
24.8% of patients underwent an abdominal surgery related to IBD after a mean time of 5,1 3,4 years. Cumulative probabilities of surgery were 6%, 15% and 22% at 1, 3 and 5 years respectively. Abdominal surgery was significantly associated with 6-TGN level (HR=2.5, CI 95% 1.4–4.5, p=0.004) for the cut off of 230 pmol/8.108RBCs.
Conclusion: This study found no association between 6-TGN levels and clinical remission at 3 and 12 months but suggested that high 6-TGN
levels increased the rate of mucosal healing and decreased the need for surgery among IBD patients treated with combination therapy.
Rubrique de classement : Maladie inflammatoires de l’intestin thérapeutique
Mots-clés : Inflammatory bowel disease, combination therapy, 6 thioguanine nucleotide, mucosal healing
Mots-clés anglais MeSH : Inflammatory bowel disease AND combination hterapy AND 6-thioguanine nucleotides AND mucosal healing
JURY : Président : M. Jean-François BRETAGNE
Assesseurs : M. Guillaume BOUGUEN [directeur de thèse] M. Laurent SIPROUDHIS [assesseur] Mme. Marie-Clémence VERDIER [assesseur]