Does immediate loading affect clinical and patient-centered outcomes of mandibular 2 -unsplinted-implant overdenture? A 2-year within-case analysis
ABSTRACT
Objective: To provide 2-year clinical- and patient-oriented data with regard to mandibular
overdenture assisted by 2 immediately loaded unsplinted implants.
Material and Methods: In this pre-post design, Phase-I clinical trial, 18 edentate individuals
(62.4 ± 7.7 years) received a new set of complete denture. Then, following standard procedures, 3 threaded implants (OsseoSpeed TX™, Dentsply Implants, Mölndal, Sweden) were placed in the mandible in each patient, and locator abutments (Zest Anchors LLC, Escondido, U.S.A.) were inserted on the right and left side implants. The midline implant served as a control for within-patient comparison. The immediate loading was conducted within 24 hours of surgery. Data were collected at baseline (T0), 12 (T1) and 24 (T2) months after immediate loading. The clinical outcomes included implant survival rate, crestal bone level changes and implant stability. These criteria were assessed through clinical and radiographic examinations as well as resonance frequency analysis. Patient-centered outcomes included patient satisfaction and oral health-related quality of life measured using validated questionnaires. Brunner-Langer approach was used for statistical analysis.
Results: Implant survival rate for immediate loaded implants was 91.7% at 2-year follow-up.
None of the unloaded implants failed. There was no statistically significant difference at baseline and follow-ups with regard to clinical outcomes between loaded and unloaded implants. Patient satisfaction and quality of life improved (p<0.0001) from baseline to 2-year follow-up.
Conclusion: Immediate loading protocol didn’t negatively affect clinical outcomes, satisfaction
This conclusion requires confirmation by randomized control trials.
Clinical Significance' statement: Mandibular overdenture assisted by two immediately-loaded
unsplinted implants is successful treatment based on 2-year clinical and patient-based outcomes.
INTRODUCTION
Based on solid evidence, nowadays many clinicians recommend the use of mandibular 2-implant overdentures in the treatment of completely edentate individuals (1). Although this treatment is cost-effective and fulfill the satisfaction of patients (2-4), clinicians desire to reduce the patients burdens such as costs, morbidity and inconvenience associated with multiple surgical phases and multiple visits as well as the long waiting time for the final restoration. Immediate loading protocol was developed to improve patients’ satisfaction and quality of life by shortening of the rehabilitation time and faster return to oral function (5-8).
However, a recent meta-analysis of seven clinical studies showed a statistical tendency in favor of conventional loading (RD: -0.03, 95% CI -0.06 to 0.00) (9). Although the clinical outcomes for immediate loading of mandibular implant overdenture showed high one-year survival rate for threaded, micro-textured implants with diameter ≥ 3mm and an insertion torque ≥ 30 Ncm and an implant stability quotient (ISQ) value ≥ 60, there was a wide variation in implant failure rates for immediate loading of unsplinted implants, ranging from 0 to 18% (9). Furthermore, data on immediately loaded implants with locator attachment is still scarce (6). A recent systematic review on immediate-loading (10) highlighted the need for clinical research that reports
on patient-centered outcomes. In fact, grading the evidence in health science literature and translation of research into practice is based primarily on patient-centered approach (11). Therefore, the objective of this study was to provide clinical- and patient-oriented data with regard to mandibular overdenture assisted by 2 immediately-loaded unsplinted implants.
MATERIAL & METHODS
Recruitment
This Phase-I trial was conducted in the Postgraduate Prosthodontic Clinics at the Université de Montréal. Ethical approval was received from the Université de Montréal Ethical Review Board (International Clinical Trial Registration # NCT01644058).
The trial used 1-group, pre-post design. The details of the study and the 4-month patient-satisfaction (primary outcome) results have been published previously (4). In brief, to be included in the trial, patients had to: (a) be at least 18 years old; (b) be wearing complete dentures in both arches for ≥1 year; and (c) have sufficient bone in the anterior mandibular region for placement of three implants of standard diameter and length without any bone grafting. Patients were excluded if they had: (a) any medical or general relative risk factors for implant therapy; (b) any physical or psychological incapacity to complete study questionnaires; (c) incapacity to follow hygienic instructions or adhere to immediate loading protocol (ILP); or (d) inability to
achieve primary stability during implant surgery (i.e., insertion torque of < 35 Ncm or resonance frequency analysis of <60 ISQ).
Prosthetic and surgical phases were conducted by two prosthodontic residents and a board-certified periodontist, respectively. Training and calibration of the two residents were done before the data collection. The
intra-class correlation coefficient (ICC) was 0.96 and 0.99 for inter- and intra examiner reliability.
Clinical procedures
Before the surgery and by using standard prosthodontic procedures, maxillary and mandibular new complete dentures; radiological guide and surgical template were fabricated for each participant. During the surgical phase, 3 threaded implants (OsseoSpeed TX™, Dentsply Implants, Mölndal, Sweden) were placed in the interforaminal mandibular area (Ø = 4mm and L = 9 to 17 mm) at a torque ≥ 35Ncm. Immediately after implantation, the prosthetic abutments were inserted (LOCATOR® abutment, ZEST Anchors L.L.C., Escondido, U.S.A.) on the right and left side implants with a final torque
of 25 Ncm. A healing abutment was inserted on the midline implant, which
was not loaded during the trial and served as a reference for within-patient comparison with regard to change in peri-implant bone level and implant stability. Study participants were informed about the use of the third implant and consented to continue participation in the future 3-implant mandibular overdenture add-on study. The immediate loading and the conversion of the
mandibular complete denture to an overdenture was conducted within 24 hours of implant surgery (4).
Data Collection
Data collection was conducted at baseline (T0), 1 year (T1), and 2 years (T2)
after the immediate loading. Additional follow-ups for the early impact of immediate loading protocol on patient satisfaction (primary study outcome) were also conducted and reported previously (4). The clinical outcomes of interest were the implant survival rate, the peri-implant crestal bone level changes and implant stability. Implant survival was defined according to Zarb and Albrektsson criteria (12): in-situ presence of the implant in function; absence of clinical mobility of the implant; absence of a persistent form of discomfort, pain or infection; and implant position allowing the placement of a functional and aesthetic prosthesis. The peri-implant bone level measurements were assessed using direct (bone probing) and indirect (radiographic assessment) methods. Direct measurements of bone crest height were obtained at four sites around each implant (buccal, lingual, mesial and distal) using the “BB gauge” as previously described (13) (Fig. 1). Radiographic evaluation was conducted using standardized periapical radiographs (long-cone paralleling technique, standard x-ray unit operating at 70 kVp, 10 mA, and 1·5 mm, and modified standardized film holder (Rinn’XCP Anterior Holder™, Dentsply Rinn, Denstply International, York, USA). The digital images were analyzed using digital subtraction technique and Image J software (ImageJ v.1.46r, National Institutes of Health, Bethesda,
USA). The junction between the machined bevel and the top of the MicroThread on the implant was used as the reference to assess the mesial and distal peri-implant crestal bone level changes. The results were presented as a mean of these two values.
The implant stability measurement was made with a resonance frequency analyzer (Osstell™, Integration Diagnostics, Gothenburg, Sweden) at the implant level (13). The transducer was attached to the implants using a manual prosthetic torque wrench (Nobel Biocare, Zurich, Switzerland) at a torque about 10 Ncm (14). For each implant, two measurements of implant stability quotient (ISQ) were obtained and the mean value was retained for data analysis.
Patients’ satisfaction and oral-health-related quality of life were measured using the McGill denture satisfaction questionnaire (15, 16), and the Oral Health Impact Profile (OHIP-20) (17). OHIP consists of 20 items covering seven domains: functional limitation, physical pain, psychological discomfort, physical disability, psychological disability, social disability and handicap. Lower OHIP scores indicated better oral health-related quality of life.
The explanatory variables for patient-centered outcomes included socio-demographic characteristics and personality traits. The latter was measured by use of the revised NEO personality inventory validated questionnaire (18). The details and related personality traits data and results are already published (4).
The sample size estimation for the study was based on the primary outcome of interest (patient satisfaction). Based on the Wilcoxon test assuming that: a) the minimal practically important pre-post difference in the mean global satisfaction score is 25 units (based on the opinion of the experts and literature) (19-22), and b) the standard deviation of the distribution of the global satisfaction score is 35 units (21), a sample size of 18 participants would allow a power of 80% of rejecting the null hypothesis if it is indeed false at an alpha level of 5%.
Descriptive statistics and Brunner Langer approach (Mixed models for non-parametric statistics) (23) were used to estimate and to analyze the data. Mixed models for non-parametric statistics (23) was conducted to compare patients’ satisfaction, OHIP total and domains scores as well as clinical and radiographic outcomes at different time points during follow-ups. The model was also used to show the effect of the implant loss on two-year outcomes. Bonferroni adjustments were conducted for multiple comparisons at different time level. Patients’ satisfaction and OHIP change scores were computed as followed: ∆ = T0 – T1, ∆ = T1 – T2, ∆ = T0 – T2. Level of significance was set at p
≤ 0.05. Data analyses were performed using SPSS 20.0 (IBM Co., Chicago, U.S.A.) and the SAS program (SAS 9.4 for Windows, Cary, U.S.A.).
RESULTS
Clinical outcomes
From a total of 23 patients who signed the consent forms, 18 edentate individuals (11 women, 5 men; mean age 62.39 ± 7.65 years), were followed
for 2 years. Four patients were excluded from the study before the surgical phase or at surgery because of lack of implant primary stability and one patient was excluded after the immediate loading because of a medical problem unrelated to the study. From a total of 54 inserted implants, 36 were immediately loaded and 18 served as within control. All implant failures occurred within the first 4 postoperative months and none of the unloaded implants failed. During the first month after the surgery, one woman lost the left implant and another one lost the two loaded implants (Figure 2, study flow chart). The patients were not considered as dropout since they both completed the 2-year follow-up for patient-based outcomes.
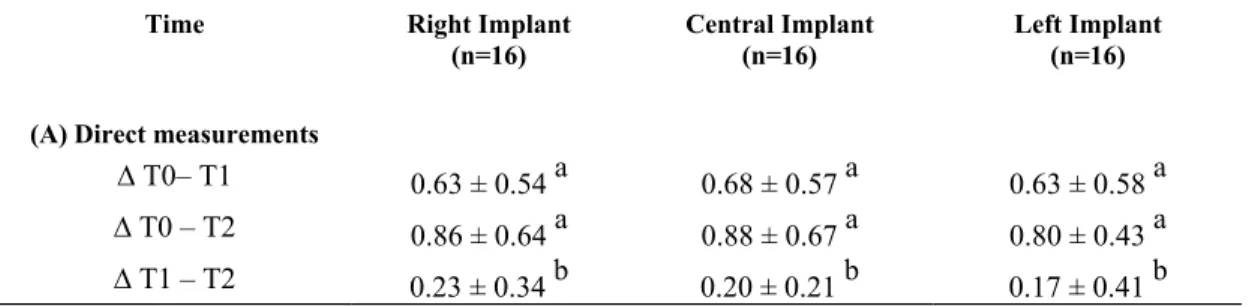
The implant survival rate at 2-year follow-up was 91.7%. There was no statistically significant difference among loaded and unloaded implant at baseline and follow-ups with regard to change in bone level and stability (Table 1 and 2). The comparison of clinical measures between follow-ups showed statistically significant peri-implant bone loss (mean value less than 1 mm) in both radiographic and direct measurements from T0 to T1 (p<0.05) and
T0 to T2 (p<0.05) for all implants. Changes from T1 to T2 (Table 1, Fig. 3)
were only statistically significant when using direct measurements technique. The implant stability quotient increased from T0 to T1 (p< 0.0001) for all
implants and was then stable from T1 to T2 (p> 0.05) (Table 2, Fig. 4).
Patient-oriented outcomes
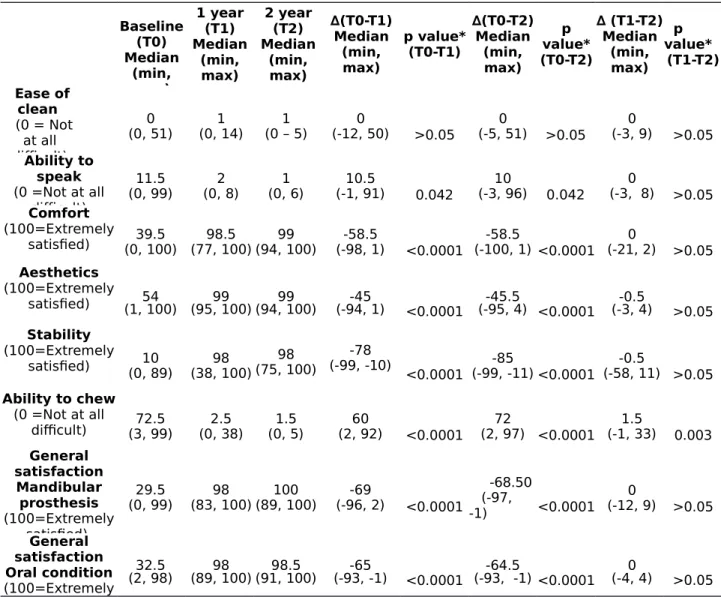
The overall general satisfaction with mandibular prosthesis increased significantly from baseline to one-year and two-year follow-ups (p< 0.0001). Similarly, the level of comfort (p< 0.0001), aesthetics (p<0.0001), lower
denture stability (p< 0.0001), ability to chew (p< 0.0001) and speak (p< 0.042), and general satisfaction of oral condition (p< 0.0001) were all significantly higher at the two follow-ups. The change scores were not statistically significant from T1 to T2 except for the ability to chew (p= 0 .003)
(Table 3).
Within-subjects analyses revealed statistically significant decreases in the total OHIP scores, and all of the domain scores from baseline to the one-year and two-year follow-ups (Table 4). There was no statistically significant difference between T1 and T2 with regard to OHIP scores (Table 4). The Brunner-Langer models showed patients’ satisfaction and oral health related-quality of life improvement regardless of implant loss (p> 0.05).
DISCUSSION
Researchers evaluating implant outcomes have used numerous approaches to measurements. A recent systematic review on implant success criteria indicated that clinical parameters could be defined as surrogate endpoints, while the real success should be considered as a composite outcome taking into account implant-prosthetic complex and patient satisfaction (24).
In this clinical trial, clinical- and patient-oriented outcomes of immediately loading process in individuals wearing a 2-unsplinted-implant mandibular overdenture were examined. For the clinical outcomes, implant survival rate, peri-implant bone loss and implant stability were reported. These outcomes could be considered as an indicator for early implant success rate since a minimum of 5-year follow-up is necessary to consider success rate as an endpoint (12). Our results showed that there was no statistically significant difference between loaded and unloaded implants for clinical outcomes, patients were highly satisfied and their quality of life improved
after 2 years of using their overdenture.
These findings are in line with previous research in regard to immediate loading of unsplinted implants. According to the recent meta-analysis, the implant survival rate in this study (91.7%) was close to the high range of existing evidence for immediate loading of unsplinted implants (9). The failure rate also was comparable to a recent study (6) that showed a failure rate of 5.5% regarding immediate loading of implants with locator attachment. As discussed in different studies, quality of bone and initial primary stability are the main predictors for the success of the immediate loading protocol (25, 26). However, in our study, although implant primary stability was attained for all study participants, one patient lost 2-loaded implants. Although not mentioned in the medical history of the patient before the implant surgery, further follow-ups with the patient revealed that this patient had age-related macular degeneration of the eye and received an ophthalmic intravitreal injection of Ranibizumab (Lucentis, Genentech Inc., San Francisco, U.S.A.) a month before surgery. Ranibizumab is a fragment of a recombinant humanized monoclonal antibody Fab (48 kDa) that inhibits the activity of human vascular endothelial growth factor, VEGF-A (27-29). VEGFs plays a crucial role in the vascular supply during the bone healing process (30, 31), and inhibiting angiogenesis may have a negative impact on bone healing and implant osseointegration (32, 33). This clinical observation lead to a further experiment (34) during which we found that ranibizumab, a VEGF inhibitor, can compromise bone healing and osseointegration by the down-regulation of angiogenesis and reducing trabecular thickness and volume. These medications that are also used in cancer therapy should be considered as potential risk factors for implant osseointegration. However, the mechanical stress applied to immediately loaded implants during the healing period and micromotions at implant/bone interface (35) may
have also facilitated the effect of that medication since the unloaded implants did not fail for that patient. Few studies have reported patient satisfaction and quality of life with immediate loading protocol of mandibular implant overdenture at different time levels ranging from one week to 6 months (36-40). Our findings support previous research that patient satisfaction is increased by immediate loading protocol. In fact, immediate and early loading protocols address patient expectations with regard to immediate restoration of function and aesthetics. To maximize patients’ satisfaction, the clinician should manage patients’ expectations by giving them detailed information about the possible treatment outcomes and complications. However, there is dearth of the literature about patients’ expectation with immediately loaded implants. Further qualitative research is needed to examine how patient experience and perceive this protocol.
The present study has some limitations. The within-case design of this this study did not permit comparison of conventionally loaded versus immediate loading implants. Moreover, all phase-I trials have a limited sample size and lack a control group. In this trial, the possible source of selection bias must be considered since the patients were recruited from a list of patients who were waiting for implant treatment at Université de Montréal.
Conclusion
Within the limitations of this study, mandibular 2- unsplinted implants overdenure with immediate loading procedure could be considered as a
successful treatment option from both clinical and patients point of view. This conclusion, yet, requires confirmation by randomized control trials.
REFERENCES
1. Emami E, Heydecke G, Rompre PH, de Grandmont P, Feine JS. Impact of implant support for mandibular dentures on satisfaction, oral and general health-related quality of life: a meta-analysis of randomized-controlled trials. Clinical Oral Implants Research 2009;20(6):533-44.
2. Awad MA, Lund JP, Shapiro SH, Locker D, Klemetti E, Chehade A, et al. Oral health status and treatment satisfaction with mandibular implant overdentures and conventional dentures: a randomized clinical trial in a senior population. International Journal of
Prosthodontics 2003;16(4):390-6.
3. Emami E, De Grandmont P, Rompré P, Feine J. Oral Health, Quality of Life and Physical Health. The International Journal of Health, Wellness and Society 2011;1(1):157-70.
4. Menassa M, de Grandmont P, Audy N, Durand R, Rompre P, Emami E. Patients' expectations, satisfaction, and quality of life with immediate loading protocol. Clinical Oral
Implants Research 2016;27(1):83-89.
5. Avila G, Galindo P, Rios H, Wang HL. Immediate implant loading: current status from available literature. Implant Dentistry 2007;16(3):235-45.
6. Elsyad MA, Elsaih EA, Khairallah AS. Marginal bone resorption around immediate and delayed loaded implants supporting a locator-retained mandibular overdenture. A 1-year randomised controlled trial. Journal of Oral Rehabiltation 2014;41(8):608-18.
7. Turkyilmaz I, Sennerby L, Tumer C, Yenigul M, Avci M. Stability and marginal bone level measurements of unsplinted implants used for mandibular overdentures: a 1-year randomized prospective clinical study comparing early and conventional loading protocols.
Clinical Oral Implants Research 2006;17(5):501-5.
8. Chrcanovic BR, Albrektsson T, Wennerberg A. Immediate nonfunctional versus immediate functional loading and dental implant failure rates: a systematic review and meta-analysis. Journal of Dentistry 2014;42(9):1052-9.
9. Schimmel M, Srinivasan M, Herrmann FR, Muller F. Loading protocols for implant-supported overdentures in the edentulous jaw: a systematic review and meta-analysis.
International Journal of Oral Maxillofacial Implants 2014;29 Suppl:271-86.
10. Gallucci GO, Morton D, Weber HP. Loading protocols for dental implants in edentulous patients. International Journal of Oral Maxillofacial Implants 2009;24 Suppl:132-46.
11. Ebell MH, Siwek J, Weiss BD, Woolf SH, Susman J, Ewigman B, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. American Family Phisicians 2004;17(1):59-67.
12. Zarb GA, Albrektsson T. Consensus report: towards optimized treatment outcomes for dental implants. Journal of Prosthetic Dentistry 1998;80(6):641.
13. Brochu JF, Anderson JD, Zarb GA. The influence of early loading on bony crest height and stability: a pilot study. International Journal of Prosthodontics 2005;18(6):506-12.
14. Ostman PO, Hellman M, Wendelhag I, Sennerby L. Resonance frequency analysis measurements of implants at placement surgery. International Journal of Prosthodontics 2006;19(1):77-83; discussion 84.
15. Awad MA, Feine JS. Measuring patient satisfaction with mandibular prostheses.
Community Dentistry and Oral Epidemiology 1998;26(6):400-5.
16. De Grandmont P, Feine JS, Tache R, Boudrias P, Donohue WB, Tanguay R, et al. Within-subject comparisons of implant-supported mandibular prostheses: psychometric evaluation.
Journal of Dental Research 1994;73(5):1096-104.
17. Allen F, Locker D. A modified short version of the oral health impact profile for assessing health-related quality of life in edentulous adults. International Journal of Prosthodontics 2002;15(5):446-50.
18. Costa PT, McCrae RR, Psychological Assessment Resources I. Revised NEO Personality Inventory (NEO PI-R) and NEO Five-Factor Inventory (NEO-FFI): Psychological Assessment Resources; 1992.
19. Al-Sunduqchi MS. Determining the Appropriate Sample Size for Inferences Based on the Wilcoxon Statistics: University of Wyoming; 1990.
20. Awad MA, Locker D, Korner-Bitensky N, Feine JS. Measuring the effect of intra-oral implant rehabilitation on health-related quality of life in a randomized controlled clinical trial.
Journal of Dental Research 2000;79(9):1659-63.
21. Michaud PL, de Grandmont P, Feine JS, Emami E. Measuring patient-based outcomes: is treatment satisfaction associated with oral health-related quality of life? Journal of Dentistry 2012;40(8):624-31.
22. Zar JH. Biostatistical analysis 2nd ed ed. Englewood Cliffs, N.J: Prentice-Hall; 1984. 23. Brunner E, Langer F. Nonparametric Analysis of Ordered Categorical Data in Designs with Longitudinal Observations and Small Sample Sizes. Biometrical Journal 2000;42(6):663-75.
24. Papaspyridakos P, Chen CJ, Singh M, Weber HP, Gallucci GO. Success criteria in implant dentistry: a systematic review. Journal Dental Research 2012;91(3):242-8.
25. Chaushu G, Chaushu S, Tzohar A, Dayan D. Immediate loading of single-tooth implants: immediate versus non-immediate implantation. A clinical report. International Journal Oral
Maxillofacial Implants 2001;16(2):267-72.
26. Chiapasco M, Abati S, Romeo E, Vogel G. Implant-retained mandibular overdentures with Branemark System MKII implants: a prospective comparative study between delayed and immediate loading. International Journal Oral Maxillofacial Implants 2001;16(4):537-46.
27. Lu F, Adelman R. Are intravitreal bevacizumab and ranibizumab effective in a rat model of choroidal neovascularization? Graefe's Archive for Clinical and Experimental Ophthalmology 2009;247(2):171-77.
28. Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. New England Journal of Medicine 2006;355(14):1419-31.
29. Schmucker C, Ehlken C, Agostini HT, Antes G, Ruecker G, Lelgemann M, et al. A safety review and meta-analyses of bevacizumab and ranibizumab: off-label versus goldstandard. PLoS
One 2012;7(8):e42701.
30. Beamer B, Hettrich C, Lane J. Vascular endothelial growth factor: an essential component of angiogenesis and fracture healing. HSS Journal 2010;6(1):85-94.
31. Ribatti D. The crucial role of vascular permeability factor/vascular endothelial growth factor in angiogenesis: a historical review. British Journal of Haematology 2005;128(3):303-9. 32. Davies JE. Understanding peri-implant endosseous healing. Journal Dental Education 2003;67(8):932-49.
33. Eriksson C, Nygren H, Ohlson K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 2004;25(19):4759-66.
34. Al Subaie AE, Eimar H, Abdallah MN, Durand R, Feine J, Tamimi F, et al. Anti-VEGFs hinder bone healing and implant osseointegration in rat tibiae. Journal of Clinical Periodontology
2015;42(7):688-96.
35. Romanos GE, Nentwig GH. Immediate versus delayed functional loading of implants in the posterior mandible: a 2-year prospective clinical study of 12 consecutive cases. International
Journal of Periodontics Restorative Dentistry 2006;26(5):459-69.
36. Borges Tde F, Mendes FA, de Oliveira TR, Gomes VL, do Prado CJ, das Neves FD. Mandibular overdentures with immediate loading: satisfaction and quality of life. International
Journal of Prosthodontics 2011;24(6):534-9.
37. Alfadda SA, Attard NJ, David LA. Five-year clinical results of immediately loaded dental implants using mandibular overdentures. International Journal of Prosthodontics 2009;22(4):368-73.
38. Attard NJ, Laporte A, Locker D, Zarb GA. A prospective study on immediate loading of implants with mandibular overdentures: patient-mediated and economic outcomes. International
Journal of Prosthodontics 2006;19(1):67-73.
39. Buttel AE, Gratwohl DA, Sendi P, Marinello CP. Immediate loading of two unsplinted mandibular implants in edentulous patients with an implant-retained overdenture: an observational study over two years. Schweiz Monatsschr Zahnmed 2012;122(5):392-7.
40. Stricker A, Gutwald R, Schmelzeisen R, Gellrich NG. Immediate loading of 2 interforaminal dental implants supporting an overdenture: clinical and radiographic results after 24 months. International Journal Oral Maxillofacial Implants 2004;19(6):868-72.
Table 1.Marginal bone level change from baseline to year 2: direct
measurements (bone probing) (A) and indirect measurements (radiographic assessment) (B)
Time Right Implant (n=16)
Central Implant (n=16)
Left Implant (n=16) (A) Direct measurements
∆ T0– T1 0.63 ± 0.54 a 0.68 ± 0.57 a 0.63 ± 0.58 a
∆ T0 – T2 0.86 ± 0.64 a 0.88 ± 0.67 a 0.80 ± 0.43 a
∆ T1 – T2 0.23 ± 0.34 b 0.20 ± 0.21 b 0.17 ± 0.41 b
No Statistically significant difference among 3 implants at T0, T1, T2 a p<.0001 b p= .0127 (B) Indirect measurements ∆ T0 – T1 0.15 ± 0.44 a 0.48 ± 0.42 a 0.42 ± 0.49 a ∆ T0 – T2 0.30 ± 0.48 b 0.47 ± 0.52 b 0.43 ± 0.47 b ∆ T1 – T2 0.14 ± 0.51 -0.01 ± 0.52 0.00 ± 0.48
No Statistically significant difference among 3 implants at T0, T1, T2 a p=0.0030
b p=0.0009
Table 2. Implant stability Quotient (ISQ value) from baseline to year 2
Time Right Implant (n=16) Central Implant (n=16) Left Implant (n=16) ∆ T0 - T1 -8.28 ± 5.88 a -7.22 ± 7.25 a -7.47 ± 5.59 a ∆ T0 – T2 -8.59 ± 5.61 a -8.59 ± 5.93 a -8.06 ± 5.22 a ∆ T1 – T2 -0.31 ± 2.54 -1.38 ± 4.23 -0.59 ± 1.82
No Statistically significant difference among 3 implants at T0, T1, T2 a p<0.0001
Table 3. Within-patient differences in pre- and post-treatment satisfaction ratings from baseline to year 2 (n=18). Baseline (T0) Median (min, max) 1 year (T1) Median (min, max) 2 year (T2) Median (min, max) ∆(T0-T1) Median (min, max) p value* (T0-T1) ∆(T0-T2) Median (min, max) p value* (T0-T2) ∆ (T1-T2) Median (min, max) p value* (T1-T2) Ease of clean (0 = Not at all difficult) 0 (0, 51) (0, 14)1 (0 – 5)1 (-12, 50)0 >0.05 (-5, 51)0 >0.05 0 (-3, 9) >0.05 Ability to speak (0 =Not at all difficult) 11.5 (0, 99) (0, 8)2 (0, 6)1 (-1, 91)10.5 0.042 (-3, 96)10 0.042 (-3, 8)0 >0.05 Comfort (100=Extremely satisfied) (0, 100)39.5 (77, 100)98.5 (94, 100)99 (-98, 1)-58.5 <0.0001 (-100, 1) <0.0001-58.5 (-21, 2)0 >0.05 Aesthetics (100=Extremely satisfied) (1, 100)54 (95, 100)99 (94, 100)99 (-94, 1)-45 <0.0001 (-95, 4) <0.0001-45.5 (-3, 4)-0.5 >0.05 Stability (100=Extremely satisfied) (0, 89)10 (38, 100)98 (75, 100)98 (-99, -10)-78 <0.0001 (-99, -11) <0.0001-85 (-58, 11) >0.05-0.5 Ability to chew (0 =Not at all difficult) (3, 99)72.5 (0, 38)2.5 (0, 5)1.5 (2, 92)60 <0.0001 (2, 97) <0.000172 (-1, 33)1.5 0.003 General satisfaction Mandibular prosthesis (100=Extremely satisfied) 29.5 (0, 99) (83, 100)98 (89, 100)100 (-96, 2)-69 <0.0001 -68.50 (-97, -1) <0.0001 0 (-12, 9) >0.05 General satisfaction Oral condition (100=Extremely 32.5 (2, 98) (89, 100)98 (91, 100)98.5 (-93, -1)-65 <0.0001 (-93, -1) <0.0001-64.5 (-4, 4)0 >0.05 VAS range: 0 mm (min) to 100 mm (max)
Table 4. Within-patient differences in OHIP-20 pre-post treatment scores from baseline to year 2 (n=18).
* Bonferroni adjusted p-value, Brunner-Langer. OHIP Subscale Baseline (T0) Median (min, max) 1 year (T1) Median (min, max) 2years (T2) Median (min, max) ∆(T0-T1) Median (min, max) p-value* (T0- T1) ∆(T0-T2)Median (min, max) p-value* (T0-T2) ∆(T1-T2)Median (min, max) p-value* (T1-T2) Functional limitation (5, 18)12.5 (3, 9)5 (3, 7)5 (1, 14)7 <0.0001 (1, 15)8 <0.0001 (-1, 4)0.5 >0.05 Physical pain (5, 23)17 (4, 12)5 (4, 9)4 (0,15)10.5 <0.0001 (1, 19)11 <0.0001 (-3, 3)0 >0.05 Psychologic al discomfort 8 (2, 12) (2, 5)2 (2, 4)2 (0, 10)5.5 <0.0001 (0, 10)6 <0.0001 (-1, 3)0 >0.05 Physical disability (4, 23)13 (4, 12)4.5 (4, 9)4 (0, 13)8 <0.0001 (0, 19)7.5 <0.0001 0 (-3, 8) >0.05 Psychologic al disability 6 (2, 11) (2, 4)2 (2, 4)2 (-1, 7)3.5 <0.0001 (0, 9)4 <0.0001 (-1, 2)0 >0.05 Social disability 5.5 (3, 13) (3, 6)3 (3, 6)3 (0, 8)2 <0.0001 (0, 8)2 <0.0001 (0, 1)0 >0.05 Handicap 5.5 (2, 12) (2, 4)2 (2, 4)2 (0, 10)2.5 <0.0001 (0, 10)3.5 <0.0001 (0, 2)0 >0.05 Total OHIP scores (24, 102)69.5 (20, 44)24.5 (20, 40)22.5 (0, 60)41.5 <0.0001 (-3, 80)44 <0.0001 (-9, 22)2 >0.05
Figure 1. Instruments used for direct measurement of peri-implant bone levels. Figure 2. Study Flow Chart (N= number of patients, ♀= female, ♂=male).
Figure 3. Change in vertical bone level: direct measurements (A) and
indirect (radiographic) measurements (B).