ContentslistsavailableatScienceDirect
Behavioural
Brain
Research
j o ur na l h o me p a g e :w w w . e l s e v i e r . c o m / l o c a t e / b b r
Research
report
Cerebral
responses
and
role
of
the
prefrontal
cortex
in
conditioned
pain
modulation:
an
fMRI
study
in
healthy
subjects
Volodymyr
B.
Bogdanov
a,b,∗,
Alessandro
Viganò
b,c,
Quentin
Noirhomme
d,e,
Olena
V.
Bogdanova
f,
Nathalie
Guy
g,
Steven
Laureys
d,e,
Perry
F.
Renshaw
f,
Radhouane
Dallel
g,1,
Christophe
Phillips
d,h,1,
Jean
Schoenen
b,1aINRA,NutritionetNeurobiologieIntégréeandBordeauxSegalenUniversity,UMR1286,146rueLéo-Saignat,BordeauxCedex,33076,France bHeadacheResearchUnit,DepartmentofNeurology,CHRCitadelle,UniversityofLiège,Liège,Belgium
cSAMILAL-Dept.Anatomy,Histology,ForensicMedicine,Orthopaedics-LaSapienza,UniversityofRome;Dept.ofNeurologyandPsychiatry-LaSapienza,
UniversityofRome
dCyclotronResearchCentre,UniversityofLiège,Liège,Belgium
eComaScienceGroup,DepartmentofNeurology,UniversityandUniversityHospitalofLiège,Liège,Belgium fUniversityofUtah,SaltLakeCity,USA
gClermontUniversité,Universitéd’Auvergne,BP10448,F-63000Clermont-Ferrand;Inserm,UMR1107,TrigeminalpainandMigraineF-63000
Clermont-Ferrand;CHU,Clermont-Ferrand
hDepartmentofelectricalengineeringandcomputerscience,UniversityofLiège,Liège,Belgium
h
i
g
h
l
i
g
h
t
s
•Themechanismsunderlyingconditionedpainmodulationaremultifaceted.
•Duringcold,application-specificcerebralactivationswerefoundinprecuneusandleftinsula. •Conditionedsuppressionofinsulatest-painresponsewasrelatedtohypoalgesia.
•Theinsularchangesarepredictedbyearlyprefrontalresponsetocoldconditioning. •Smallerearlyprefrontalresponsepredictsheterotopicnoxioushyperalgesia.
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received27June2014
Receivedinrevisedform28October2014 Accepted18November2014
Availableonline25November2014 Keywords:
Brainimaging Prefrontalcortex
Conditionedpainmodulation
a
b
s
t
r
a
c
t
Themechanismsunderlyingconditionedpainmodulation(CPM)aremultifaceted.Wesearchedforalink betweenindividualdifferencesinprefrontalcortexactivityduringmulti-trialheterotopicnoxiouscold conditioningandmodulationofthecerebralresponsetophasicheatpain.
In24healthyfemalesubjects,weconditionedlaserheatstimulitothelefthandbyapplying alter-nativelyice-coldorlukewarmcompressestotherightfoot.Wecomparedpainratingswithcerebral fMRIBOLDresponses.WealsoanalyzedtherelationbetweenCPMandBOLDchangesproducedbythe heterotopiccoldconditioningitself,aswellastheimpactofanxietyandhabituationofcold-painratings. Specificcerebralactivationwasidentifiedinprecuneusandleftposteriorinsula/SII,respectively,during earlyandsustainedphasesofcoldapplication.Duringcoldconditioning,laserpaindecreased(n=7), increased(n=10)orstayedunchanged(n=7).Attheindividuallevel,thepsychophysicaleffectwas directlyproportionaltothecold-inducedmodulationofthelaser-inducedBOLDresponseinleftposterior insula/SII.ThelattercorrelatedwiththeBOLDresponserecorded80searlierduringtheinitial10-sphase ofcoldapplicationinanteriorcingulate,orbitofrontalandlateralprefrontalcortices.
Abbreviations: ACC,anteriorcingulatecortex;BOLD,bloodoxygenationlevel-dependentresponse;fMRI,functionalmagneticresonanceimaging;FWER,family-wise errorrating;NRS,numericratingscale;OFC,orbitofrontalcortex;PAG,periaqueductalgraymatter;PFC,prefrontalcortex;SMA,supplementarymotorarea;SIandSII, primaryandsecondarysomatosensorycortices;VOI,volumeofinterest.
∗ Correspondingauthorat:BordeauxSegalenUniversity,146rueLéo-Saignat,BordeauxCedex33076,France.Tel.:+33557571226;fax:+33557571227. E-mailaddress:vlabogd@yahoo.com(V.B.Bogdanov).
1 Lastauthorscontributedequallytothesupervisionofthestudy.
http://dx.doi.org/10.1016/j.bbr.2014.11.028
1. Introduction
Endogenouspaincontrolisacomplexmulti-stepprocess com-posedofseveralinteractingneuronal systemsand mechanisms: stimulation-inducedanalgesia[1],diffusenoxiousinhibitory con-trols[2],ascendingnociceptivecontrol[3]andplaceboanalgesia [4],distractionordisengagement[5,6],stress-inducedanalgesia [7],and“offset”analgesia[8,9].
Diffuse noxious inhibitory controls (DNIC) are a form of supraspinal endogenous analgesia where phasic test pain responses are attenuated by heterotopic noxious conditioning stimuli.
Theterm“conditionedpainmodulation”(CPM)wasproposed todefine thepsychophysicalparadigm totest DNIC [10,11]. In humans,theconditioningstimulusmodulatingtheresponsetothe teststimuluscanbepainfulornon-painfulandCPMcaninhibitor facilitatethisresponse[11].CPMcanbeassociatedwithchanges inneuronalactivityatmultiplesitesfromspinalcorddorsalhorn, medullarynucleusreticularisdorsalis,periaqueductalgraymatter [12–14]toprefrontalcortex[15,16]
UnderstandingthemechanismsinvolvedinCPMisimportant because it is impaired in several hyperalgesic conditions (see forareview[17]:tension-typeheadache[18],migraine[19,20], fibromyalgia[21,22],irritablebowelsyndrome[23],osteoarthritis [24]andchronicpost-surgicalpain[25].
Functionalneuroimagingstudieshaveshownthatactivationsof primarysomatosensory(SI),prefrontal(PFC)oranteriorcingulate cortices (ACC) are associated with conditioned pain modula-tion [15,16,26]. The PFC, for instance, is activated in healthy controls by heterotopic cold stimulation during rectal pain [23,27]. Sustained cold-induced responses in orbitofrontal cor-tex (OFC)are linked tocold-induced analgesia [15]. Functional couplingbetweenthesubgenualanteriorcingulatecortex(ACC) and PAG is related to stronger individual heterotopic noxious analgesia[16].
Themechanisms of endogenous analgesia are clearly multi-facetedanddespitethenumerousstudiesinhealthysubjectsand inpathologicalconditions,thepreciseunderlyingneuralprocesses arestilluncertain.Moreover,painisahighlysubjectiveexperience andcanbeinfluencedbydifferencesinindividualsusceptibilityas wellaspersonality.Theaimofourstudywasthereforetosearchfor alinkbetweenindividualdifferencesinactivityofcerebralregions ofinterestduringmulti-trialheterotopicnoxiousconditioningand modulationoftheresponsestotestpainstimuli.
Toassessbrainactivity,weanalyzedfMRIBOLDresponses pha-siclaserheat-inducedtestpainwithorwithoutsustainednoxious coldconditioningandtheircorrelationwithself-ratingsofpain lev-els.Wefocusedonthemodulationoftest-painresponsesinthe so-called“painmatrix”[28]andthelinksbetweentheindividual differencesinCPMandprecedingorconcurrentneuronalresponses tonoxiouscoldconditioninginprefrontalareas(ACC,lateralPFC andOFC).
Contrarytotheabove-mentionedstudies,weusedarepeated block paradigm of noxious cold conditioning alternating with lukewarm application, which allows identifyingmore precisely
responsesinPFCtonoxiouscoldstimulationandreducesnovelty effects.
We expected that continuous cold pain stimulation would reduceself-ratingsoflaserheat-inducedpainandassociated cere-bralresponsesinthepainmatrix.InthoseareasofPFCknownfrom previousstudiestobeinvolvedindescendinganalgesia(ACC, lat-eralPFCandOFC),wesearchedforcold-inducedactivation,butalso forpossibledeactivation,asreportedinACC[29]andPFCareas[30]. WehypothesizedthatindividualdifferencesinPFCresponsesto coldpainwouldcorrelatewithlevelsofCPM.
2. Materialandmethods 2.1. Subjects
Twenty-fourhealthyfemalevolunteers(meanage:31.3years, range:24–45years)tookpartinthemainexperimentandfour subjectsparticipatedinapilotstudy.
Noneofthesubjectswassufferingfrompain,neurologicalor psychiatricdisorders.Allsubjectssignedaninformedconsentform thatmentionedthegeneralobjective,i.e.tostudytheimpactofa coldstimulusonpainandphysiologicalbrainresponsesinducedby laserheatstimuli,andwerethusnaïveaboutourworking hypothe-sis.Theyreceivedafinancialcompensationfortravelexpensesand timespentinthelaboratory.TheEthicsCommitteeoftheUniversity ofLiègeapprovedthestudy.
2.2. Experimentalprotocol
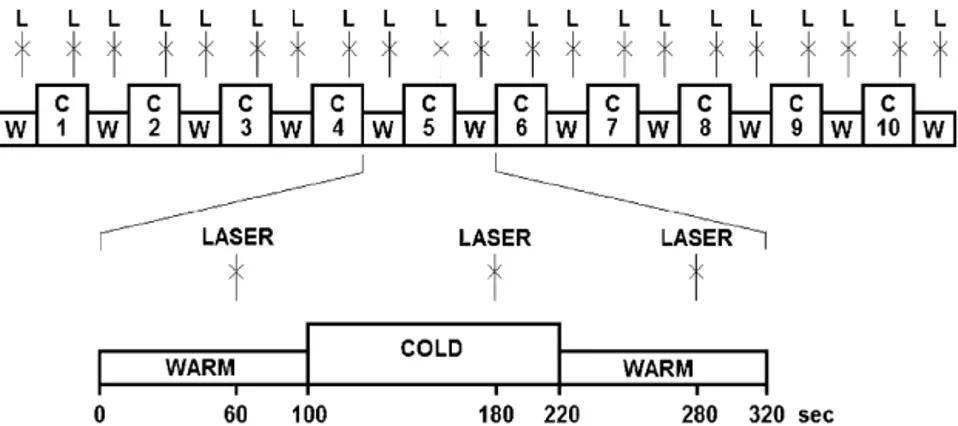
Laserheatpulsesdeliveredtothedorsumofthelefthandserved aspainfulteststimuli,whereassustainednoxiouscoldappliedon therightfootwasusedastheconditioningstimulus.Tenblocks ofcoldstimulation(120s)alternatedwith11blocksoflukewarm stimulation(100s).Singlelaserstimuliweredeliveredonce40s beforetheendofeachblock(Fig.1).
Fortheconditioningstimulation,weusedanapproachsimilar tothatofSprengeretal.,whoused12smallbags(600goficeand 250mlofwater)tocoverthewholesurfaceofthelegandthefoot [16].Weappliedtwolargerbags(2L ofcrushediceand200ml ofwater)tothefootonly.Therewereseveralbagspreparedin advance.Theywerekeptinfoamboxeswhennotappliedtothe sub-ject.Besidescompressbagssomeadditionalicewasalwayspresent inthecontainer.Wecontrolledthetemperatureoftheicebags.In general,thetemperatureinCPMexperimentsisadjustedbya cir-culationcircuit(5–8◦C)[31,32]orastillicewaterbath(4–6◦C) [15,33]suchastobetolerable.Inourexperiment,thetemperature wasabout+2◦Canddidnotchangesignificantlythroughoutthe experiment.Thiswasachievedbyalargeproportionoficeinthe bagsandthestoragecontainer,whichmaintainedalow temper-atureduringthemeltingprocess.Ourmodeofcoldconditioning resultedinarelativelylesspowerfulnoxiousstimulation,butall oursubjectswereabletotoleraterepetitive2-minnoxiouscold applicationsduringtheentireexperimentalsession.
Thesameinvestigator(A.V.)manuallyappliedthecompresses overtherightfootofthesubjectsinthescannerroom.
Fig.1.Experimentalprotocol.Continuouslukewarm+35◦C(W)andcold2±2◦C(C)stimulationisappliedtotherightfootofthesubjects.Attheendofeachlukewarmand
coldblock,asinglelaserpulse(L)isappliedtothedorsumofthelefthand.
Laserpulses(testpain)weregeneratedbyathuliumYAGlaser (BaaselLasertech,Starnberg,Germany)thatemitscalibrated near-infraredradiation(wavelength1.96m;spotdiameter5mm;pulse duration1ms,energy600mJ).Tofamiliarizethesubjectswiththe procedure,threelaserpulseswereadministeredbeforethe begin-ningoftheexperiment.ThisexperimentwasrealizedusingCogent 2000(Cogent2000teamandCogentGraphicsdevelopedbyJohn RomayaattheWelcomeDepartmentofImagingNeuroscience). 2.3. Behavioraldataacquisition
BeforethefMRIstudy,thesubjectsfilledintheFrenchversionof Spielberger’straitanxietyinventory[34,35].Theywereinformed thattheyshouldbeattentivetothelevelofpainproducedbythe heatstimulusonthehandtheywouldhavetorateafterthe exper-imentalsession. Painratingswereperformedinpenandpencil formatonanumericratingscale(NRS)from0for‘nopain’to10for ‘worstimaginablepain’.Painratingsforlaserstimuliwereobtained separately during lukewarm and cold conditioning.“Behavioral CPM”wasdefinedasadifferenceofatleastonepoint between laser-painratingsduringcoldandlukewarmconditioning.
Subjectsratedcold-inducedpainforthe1standthelast con-ditioning, each time at onset and offset of the cold compress application.Theaverageofthesefourratingswastakenas mea-sureofcold-inducedpain.Habituationofcold-inducedpainwasthe differencebetweenratingsduringthe1standthelastapplication. 2.4. fMRIdataacquisitionandanalysis
MRIdatawereacquiredona3Thead-onlyscanner (Magne-tomAllegra,SiemensMedicalSolutions,Erlangen,Germany)using thestandard transmit-receivequadratureheadcoil.Astructural MRI was obtained in a separate session 20–30min beforethe functionalacquisition,usingahigh-resolutionT1-weightedimage [36]. Multislice T2*-weighted functional images were acquired with a gradient-echo echo-planar imaging sequence using an axial slice orientation and covering the whole brain (repeti-tion time=2040ms, echo time=30ms, flip angle=90◦, field of view=192×192mm,voxelsize3×3×3mm,34slices,25% inter-slicegap,matrixsize64×64×34).Thethreeinitialvolumeswere discardedtoavoidT1saturationeffects.Fieldmapdatawerealso acquiredtounwarpthefunctionalimages:acquisitiontime:TR/TE: 517/4.92&7.38ms, flip angle90◦, field of view: 220×220mm2, resolution:64×64,voxelsize:3.4×3.4×3mm,slices=32(3mm thick,30%gap;bandwidth:260Hz/Px).
ThefMRI imageswereprocessed usingthe“Statistical Para-metric Mapping” software (SPM8; Wellcome Trust Centre for Neuroimaging,UniversityCollegeLondon,UK;http//www.fil.ion. uce.ac.uk/spm)implementedinMATLAB(MathworksInc., Sher-born,MA,USA).Functionalimagetimeserieswerecorrectedfor
motionanddistortionusingthe“RealignandUnwarp”tool[37] togetherwiththeFieldmapToolbox[38].Thehigh-resolutionT1 image was co-registered with the functional images and seg-mentedintograymatter,whitematterandcerebrospinalfluid[39]. FunctionalimageswerespatiallynormalizedtotheMontreal Neu-rologicalInstitute(MNI)space(voxelsize:3×3×3mm)usingthe normalizationparametersobtainedfromthesegmentation pro-cedureand,subsequently,smoothedwithaGaussiankernelwith full-widthathalf-maximumof6mm.
2.5. Statistics
Dataanalysiswasperformedusingagenerallinearmodeland summarystatisticsapproach.
2.5.1. BOLDresponsestolaser-heatstimulationandtheir modulationbynoxiouscold
Atthefirstlevel,dataweresubjectedtohigh-passfilteringusing acut-offperiodof280s.Eachboxcarorimpulsestimulusfunction wasconvolvedwithacanonicalhemodynamicresponsefunction asimplementedinSPM8. Explicitmaskswerecreatedfromthe brainmaskimageavailableinSPM8.Thebinarymaskwascreated bythresholdingthebrainmaskimageatalevelof0.5intensity units.Thismaskfittedtheaverageanatomicalimageofoursubjects’ groupwell.
Preliminary experiments in four subjects with sustained cold/lukewarm stimulation onthe foot showed that the onset ofbothstimulationmodalitiesinducedhighamplitudebut tran-sientBOLDresponseswithmaximaindorsal SIcontralateral to thestimulatedside.Inordertodiscriminatethisearlyresponse, eachcoldconditionwasmodeledasthreesuccessiveblocks, last-ing10sfor“early”,10sfor“transitional”and100sfor“sustained” cold responses.For thesustainedlukewarm condition,weonly modeledthe1st10sfor“early”lukewarmandthe2nd10sfor “sec-ondary”lukewarmresponses.Thelaserresponsesweremodeledas instantaneouseventswithseparateregressorsforthoseoccurring duringcoldandthoseoccurringduringlukewarmstimulation.An autoregressivemodeloforder1,pluswhitenoise,accountedfor correlationoftheresiduals.
Aftermodelestimation,specificeffectsweretestedwith appro-priatelinearcontrastsoftheparameterestimates,resultingina contrastvaluefor eachvoxel.We computedthefollowing con-trasts:(1)earlynon-specificBOLDresponsestolukewarmorcold applications; (2) early cold pain responses vs early lukewarm responses; (3) sustainedcold responses; (4) laser pain-induced responsesduringlukewarmcondition,asa measureofbaseline testpain-inducedcerebralchanges,(5)cold-inducedmodulationof laserresponses,i.e.changeofthelaser-inducedresponsesduring coldapplicationascomparedtothoseinthelukewarmcondition.
Fig.2.Sequentialoutlineofthestatisticalanalysesusedinthestudyanddefinitionofterms.
The contrast imageswere included in a second-level group analysisusingarandom-effectsapproach, treatinginter-subject variabilityasarandomfactor.Group-leveleffectsweretestedusing one-sidedt-tests.
2.5.2. CorrelationbetweenbehavioralCPMandmodulationof laserpain-inducedBOLDresponses
Therelationbetweennoxiouscoldconditioningoflaser-evoked cerebralresponsesandbehavioralCPMassessedwiththeNRSwas furthertestedinthesecond-levelgroupanalysis.Themodulation oflaserresponses(contrast5)andthebehavioralanalgesiaratings werecomparedinabetween-subjectcorrelationanalysis(Fig.2). Thisallowedfindingbrainareas where cold-induced changeof thelaserpain-inducedresponsewasdirectlycorrelatedwithpain ratings,i.e.behavioralCPM.Thisregionalbehaviorallycorrelated modulationoflaser-induced neuronalresponseswasdefinedas “physiologicalCPM”.
2.5.3. Correlationbetweencold-inducedresponsesinprefrontal cortexandphysiologicalCPM
To verify our hypothesis on the relationship between cold-inducedphysiologicalCPMandcold-inducedresponsesinPFCareas, weanalyzedthecorrelationbetweenearly,sustained(negativeand positive)coldpainresponses(contrasts2and3)andphysiological CPM(seeFig.2).
Thevolumeofinterestwasderivedfromthestatisticalmapof physiologicalCPM.Clusterswereformedbythresholdingthevoxel statisticsatp<0.001uncorrected.Subsequently,thelargestcluster withinthe“painmatrix”wasbinarizedtocreateastudy-specific mask.ThismaskcoveredtheareaswherephysiologicalCPMwas observed.Theeigenvariatesofindividualcontrastimagesof cold-inducedmodulationoflaserresponses(contrast5)wereextracted fromabrainareaoutlinedwiththemaskofphysiologicalCPMand usedascovariateregressorsinfurthercorrelationanalyses,in par-ticular,inaone-samplet-testofindividualimagesofcontrasts2 and3(earlyandsustainedcoldresponses).
2.5.4. Significancelevelanddirectedsearchforpainresponses andpainmodulation
First,weusedanundirectedsearch,lookingforresponseswith p<0.05familywiseerrorrate(FWER)correctedforthewhole-brain volumebothatvoxelandclusterlevels.Fortheclusterlevel infer-ence,wefirstuseda cluster-formingthresholdofp<0.001(not corrected).Significantresultsfromthisundirectedsearchare indi-catedinthetextandinthetablesby*WB(wholebrain).Hereandin otheranalyses,wetookintoaccountbothpeak-andcluster-level significanceandlabeledthemas*WB,respectively,nexttopeak coordinatesandclustersize.
Second,weperformedadirectedsearchfocusingonvolumesof interest(VOI).Weapplieda“smallvolumecorrection”inorderto obtainFWER-correctedp-valuesatbothvoxeland clusterlevels (withacluster-formingthresholdofp<0.001).“Painmatrix”VOI includedbrainstem,thalamus,striatumandcorticalareasknown torespondtonoxiousstimulation(seeSupplementaryMaterial1). Significantresultsforlaserandcoldresponsesin“Painmatrix”VOI
werelabeledwith*PM.Whenalargeclusterencompassing multi-plebrainareaswasfound,itwasmaskedwiththepainmatrixto identifyspecificbrainareasincluded.WedefinedfivebilateralVOI includingPFCareasmostoftenreportedtobesourcesof descen-dingCPMeffects:medialOFC,lateralOFC,ACC,rostrallateralPFC andcaudallateralPFC(seeSupplementaryMaterial2).Thosefive masksofPFCVOIwereusedforcoldresponsesandthecorrelation analysisofcoldresponsesandphysiologicalCPM.Significantresults ofthisdirectedsearchwereindicatedwith*DS(directedsearch).
Finally,smallvolumecorrectionwasappliedtospheresaround physiologicalCPMpeakswitharadiusof10mm(seeTable2)to test whethertheindividualratingsofanxietyor across-session coldhabituationcouldinfluencetheabove-mentionedresponses. Significantresultsoftheseanalyseswerelabeled*SV(small vol-ume).Pearson’scorrelationwasestimatedfortherelationbetween behavioralandphysiologicalvariables.
3. Results
3.1. BehavioralCPM
Althoughbothlaserstimuliandcoldapplicationproducedpain, thelatterwasperceivedasmorepainfulontheNRSthantheformer (5.6±0.4vs3.8±0.3,respectively,mean±SEM)(Fig.3).
Wefoundnosignificantdifferenceinaverageratingsof laser-inducedpainbetweenthelukewarmandcoldconditions,e.g.no significantmean cold-induced behavioralCPM. Thiswasdue to thefactthat,attheindividuallevel,7subjectshadhypoalgesia duringcoldconditioning(NRSreductionof≥1),10subjectshad hyperalgesia(increase of ≥1) and 7 subjects had equal ratings oflaser-induced painduring thelukewarm and coldconditions (Fig.3).
3.2. CerebralBOLDresponses
3.2.1. Non-specificcerebralresponsestoconditioningstimuli Non-specific responses to the application on the right foot of eithercold orlukewarm compresseswere significant(FWER p<0.001voxellevel)in bilateral,but predominantly left,SI,SII, SMA,thalamus,insula, precuneus,ACC,MCCanddorsal premo-torcortex,aswellasin centralcerebellum,midbrain(including PAG),bilateralamygdala,predominantlyrighthippocampusand dorsolateralPFC.
Lesssignificantactivationwasalsofoundincaudatenucleus bilaterally, left putamen, frontal operculum and cuneus (FWER p<0.05voxellevel)(seeSupplementaryMaterial3).
3.2.2. Specificcerebralresponsestoearlycoldstimulation
Atthewholebrainlevel,whenonlytheresponsetothefirst10s ofcoldstimulationwasconsideredandcontrastedtothatduring thefirst10soflukewarmstimulation,wefoundsignificant activa-tioninalargeclusterincludingbilateralprecuneus,dorsalposterior cingulateandpredominantlyleftoccipitalcortex(1259voxelsor 34cm3,p<0.005FWERclusterlevel)(Table1AandFig.4).In addi-tion,threeothersignificantlylargeclusters(p<0.05FWERcluster
Fig.3.Distributionofscoresonanumericratingscale(NRS,0-10)fornoxiouspain anditsmodulationbythermalconditioning.Thetotalnumberofsubjects(abscissa) is24.(A)Coldpainscores.(B)Laserpainscoresduringlukewarmstimulation.(C) Variationoflaserpainscoresduringnoxiouscoldstimulationexpressedasthe differencebetweenindividuallaserpainscoresduringlukewarmandcold con-ditioning.Positivevaluesrepresentcold-inducedanalgesiawhilenegativevalues indicatecold-inducedhyperalgesia.
level)encompassedlefttemporo-occipitalcortex,rightdorsal pre-motor/motorcortexandrightputamen/amygdala.
UsingthedirectedsearchwithinVOIofthe“painmatrix”,the precuneussignalwasconfirmedtobesignificant(p<0.05FWER voxellevel)and significantclusterswereidentified inpremotor cortexandventralposteriorcingulate(bothp<0.05FWERcluster level).InprefrontalVOI,therewerethreesmall,butsignificant clus-tersinmedialOFCandrightcaudo-lateralPFC(Table1AandFig.4).
Table1
Brainareasshowingaresponsetonoxiousstimuli.
Clustersize(kE) andclusterlevel significance Peaklocation ([X,Y,Z]inMNI coordinates)and voxellevel significance 1A.Earlynoxiouscold-inducedBOLDresponses
Precuneus [–3–7349]*PM
Temporo-occipitalcortex 152*WB [–57–70–75]
Dorsalpremotor/motorcortex 124*WB(#65*PM) [45558]
Caudalputamen,amygdala, hippocampus
119*WB [27–4–8]
Dorsalposteriorcingulate #72*WB [6–4046]
Ventralposteriorcingulate #68*PM [3–4316]
MedialOFC 7*DS [1247–11]*DS
8*DS [–929–17]*DS
Caudo-lateralPFC 10*DS [482328]*DS
1B.Sustainednoxiouscold-inducedBOLDresponses Whitematter,caudalcorona
radiata
15*WB [–21–2525]*WB
Caudatetailandwhitematter adjacenttoit
5*WB [18–1628]*WB
1*WB [–18–125]*WB
1*WB [–18–1328]*WB
Whitematter,spleniumof corpuscallosum
3*WB [12–3713]*WB
Posteriorinsula 4*WB [–33–2219]*WB
Largecaudalwhitematter cluster
3350*WB [–21–2525]*WB
Rostro-dorsalthalamus #102*WB [–12–1919]
1C.Sustainednegativenoxiouscold-inducedBOLDresponse
MedialOFC 11*DS [–1229–17]*DS
7*DS [335–17]
1D.Laserpain-inducedresponses
Insula/frontaloperculum 514*WB [3617–2]*WB 79*WB [–3020–5]*WB 17*WB [45–1616]*WB 18*WB [–39–7-2]*WB 2*WB [–30147]*WB 1*WB [42–710]*WB Premotor #29*WB [481119]*WB Amygdala #10*WB [18–4–14]*WB #4*WB [–21–4–14]*WB ventralputamen 64*WB [–158–11]*WB anteriorthalamus 28*WB [924]*WB PCC 26*WB [3–2231]*WB SII 25*WB [–60–2822]*WB 1*WB [63–2222]*WB ACC 19*WB [02025]*WB angularcortex 18*WB [–60–4943]*WB 4*WB [33–4943]*WB 6*WB [60–4037]*WB Dorso-lateralPFC 9*WB [48387]*WB Hippocampus 2*WB [–15–34-8]*WB #48*PM [–18–7–14]*WB Caudo-ventralthalamus 1*WB [6–284]*WB Thalamus 1*WB [12–1010]*WB SI 1*WB [54–1016]*WB OFC 1*WB [2729–17]*WB Precuneus 287*WB [12–6431]*PM Rostro-lateralPFC 255*WB [–483819] 81*WB [365331] SMA #215*WB [61161]*PM midbrain #231*WB [0–31–5]*PM
Middletemporalgyrus 60*WB [–60–587]
Cerebellum 52*WB [–18–73–32]
57*WB [12–91–26]
Caudatenucleus #8 [–984]*PM
#24 [957]*PM
Note:WB,wholebrainvolumecorrectedundirectedsearch,*PM,“painmatrix”VOI directedsearch;*DS,directedsearchatfivesmallerPFCandACCVOI.Ifindicated nexttoclustersizeornexttopeakcoordinatepointsatclusterlevelandvoxellevel FWER<0.05significance;#,inclusiveanatomicalmaskwasappliedtostatistical imageinordertoseparatesignificantresponseinasmallbrainareawhichisapart ofalargercluster.
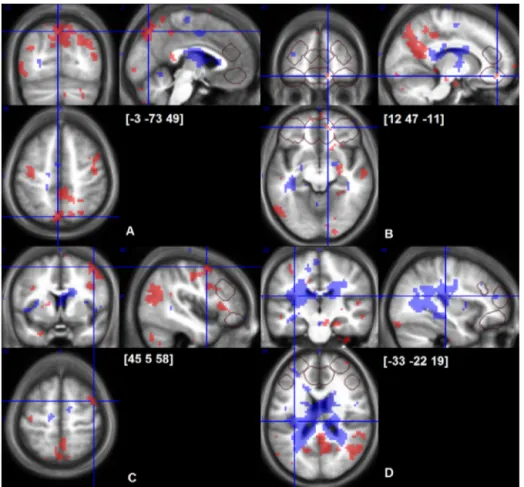
Fig.4. Cerebralresponsestoearly(shadedareaswithbackdots)andsustained(shadedareaswithwhitedots)cold-inducedpain.p<0.001uncorrectedmapsoverlaidon averageofindividualnormalizedstructuralT1images(n=24).Crosshairsarelocatedatpeaksofearlycold-painresponseinprecuneus(A),OFC(B),dorsolateralprefrontal cortex(C)andapeakofsustainedcoldpainresponseinposteriorinsula(D).VOIsofdirectedsearchareoutlinedwithgreyframes.
3.2.3. Specificcerebralresponsestosustainedcoldstimulation
SustainedcoldstimulationinducedsustainedBOLDresponses
inonlyafewclustersmostlyextendingintoposteriorwhite
mat-ter(Table1B).Responsesweresignificantinposteriorinsulaand tailofcaudate(FWER<0.05 voxellevel).Thosepeakswerepart ofalargesignificantwhitemattercluster(30cm3,p<0.001FWER clusterlevel)(Fig.4).Whenmaskingwiththe“painmatrix”VOI wasapplied,this30-cm3clusterincludedasignificantlargecluster inrostro-dorsalthalamus(p<0.01FWERclusterlevel)(Table1B). None of thedirected searches in prefrontal cortex VOI yielded significantsustained positiveresponsestocoldstimulation, but negativesustainedcold responses weredisclosedin mOFC VOI (Table1CandFig.5).
3.2.4. Laser-inducedcerebralresponsesduringlukewarm stimulation
Laserheatstimuliinducedasignificantresponseinmostareas ofthe“painmatrix”mask(seeSupplementaryMaterial1):insula, premotorcortex,amygdala,ventralputamen,anteriorthalamus, PCC,SII,ACC,and dorso-lateralPFC(peak levelFWERp<0.005) (Table1D).Outside of the“painmatrix”,there weresignificant laser-inducedresponsesbilaterallyinangulargyrusand cerebel-lum.
3.2.5. Changesinlaser-inducedcerebralresponsesduringcold stimulation
Theoveralldifferenceinresponsestolaserstimuliwithor with-outcontinuouscoldapplication(contrast 5)wasnotsignificant, whichwasduetothecontrastingCPMeffectbetweensubjects. Individualdifferencesincold-inducedbehavioralCPMscoreswere
infactproportionaltothecold-inducedchangeinlaser-induced responsesintherightanteriorinsularclusterpartiallyincluding whitematter,ventralpremotorcortexanddorsalstriatum.A sim-ilarrelationshipwasfoundinanotherclustercomprisingmostly
Fig.5. NegativeresponsestosustainednoxiouscoldstimulationinmedialOFC (out-linedwithwhite).Suprathreshodp<0.001uncorrectedT-maps.VOIsofdirected searchareoutlinedingrey.
Table2
PhysiologicalCPM:Coldmodulationoflaser-inducedresponsescorrelatedwith behavioralCPM.
Clustersize(kE) andclusterlevel significance Peaklocation ([X,Y,Z]inMNI coordinates)and voxellevel significance Anteriorinsula/white matter/premotor cortex 122*WB [302616]*WB Posteriorinsula/SII 90**WB [–36–1619]*WB LateralOFC 64*WB [2414–20] Fusiform gyrus/parahippocampal 84**WB [–21–52–11] Posteriorinsula/SII 58**PM [–36–1619]*PM
Note:seeTable1.
graymatterwithintheanatomicallydefined“painmatrix”mask: leftposteriorinsulaandSII.Thereweretwoothersignificantbut smallerclustersinlateralOFCandfusiformgyrus/parahippocampal cortex(Table2).However,whenwe appliedsmallvolume cor-rectionstothe“painmatrix”VOI,onlytheleftposteriorinsula/SII clustersurviveddirectedsearch(Table2andFig.6).
We used therefore the insula/SII cluster in the “pain matrix” (cluster size=58 voxels) to extract the eigenvariates of cold-inducedmodulationof laser responses(contrast 5). The cold-induced change of laser-induced responses in this cluster was directlycorrelated with individual behavioral CPM ratings. As mentioned, we called “physiological CPM” this concordant behavioral-BOLDresponsemodulation(Fig.2).
3.2.6. Correlationwithspecificcold-inducedprefrontalcortex responses
ThephysiologicalCPMparameterwasusedasacovariate regres-sorinaone-samplet-testofindividualimagesofcontrasts2and3 (earlyandsustainedcoldresponses).Thistestedthehypothesis thataspecificpatternofcold-inducedresponsesinPFCpreceeds subsequentphysiologicalCPMandhencemayexplaintheindividual differencesinCPM(seeFig.2fortheanalysisflow).When behav-ioralCPMratingswereincludedasacovariate,weobtainedsimilar
Fig.6.CerebralareasthatwereactivatedbytheLaser-teststimulationduring luke-warm,i.e.control,conditioning(shadedareawithblackdots)orsustainedcold conditioning(shadedareawithlight-greydots)andareaswherecold-induced inhi-bitionoflaserresponsescovariatedwithindividualanalgesiaratings(“physiological analgesia”,outlinedinwhite).p<0.001uncorrectedmapsareshownona sag-ittalsectionofaverageofindividualnormalizedstructuralT1images(n=24)at X=−36mmMNI.Crosshairlocationisatthemaximumofgreencontrast([–36–16 19]*PM).
Table3
Brainareaswherecold-inducedBOLDresponsesco-variatewithphysiologicalCPM (sourceofthephysiologicalCPMcovariateinleftinsula/SII).
Clustersizeand clusterlevel significance
Peaklocation ([X,Y,Z]inMNI coordinates) andvoxellevel significance 3A.Earlycold-inducedBOLDresponsesrelatedtophysiologicalCPM
Dorso-lateralPFC 29*WB [–155025]*WB 4*WB [–212531]*WB RostralACC 18*WB [94410]*WB 3*WB [–94116]*WB CaudalACC 12*WB [–122931]*WB Anterior-laterallingual/medial fusiformgyrus 11*WB [–24–55-2]*WB MedialPFC 8*WB [65616]*WB
Whitematter,caudalcorpus callosum
8*WB [–15–1331]*WB
Caudatehead 7*WB [–15201]*WB
Whitematterprecentralgyrus 4*WB [–36–728]*WB
Caudo-lateralOFC/rostralinsula 3*WB [–2723–14]*WB
1*WB [–3029–11]*WB 1*WB [–2411–17]WB Ventralstriatum/accumbens 2*WB [68–14]*WB SubgenualACC 2*WB [026–8]*WB Putamen 2*WB [2111–8]*WB 1*WB [27510]*WB Frontalpole 1*WB [186513]*WB 1*WB [–275910]*WB ACC 223*DS [94410]*DS Rostro-lateralPFC 178*DS [–155322]*DS MedialOFC 68*DS [635–2]*DS 11*DS [–944–8]*DS Caudo-lateralPFC 26*DS [452022]*DS LateralOFC 20*DS [–3029–11]*DS 4 [3929–17]*DS
3B.Positivesustainedcold-inducedBOLDresponsesrelatedto physiologicalCPM
Whitematter,internalcapsula, coronaradiata,putamen,insula
172*WB [–18114]
Cerebellum 146*WB [33–58–38]
Whitematteranteriorcorona radiata,adjacenttorostro-lateral PFC
117*WB [243816]
Rostro-lateralPFC 17*DS [274710]
3C.Negativesustainedcold-inducedBOLDresponsesrelatedto physiologicalCPM
LateralOFC,whitemattercorona radiata,insula,ACC,rostro-lateral PFC 229*WB [–3023–17] Cerebellum 83*WB [21–64–41] 64*WB [36–73–75] 73*WB [–27–64–41] Rostro-lateralPFC 41*DS [–244716]*DS LateralOFC 24*DS [–3023–17]*DS ACC 8*DS [–122919]*DS 5*DS [–124422] Caudo-lateralPFC 23*DS [453516]*DS
Note:seeTable1.
thoughlesssignificantresultsthatarenotpresentedbecausewe considerthemredundant.
EarlycoldresponsesindorsolateralandmedialPFC,ACC, lin-gual/fusiformgyrus,whitematteratprecentralgyrusandcaudal corpus callosum, caudo-lateral OFC/rostral insula, striatum and frontalpoleweredirectlycorrelatedwithphysiologicalCPM(FWER p<0.05 voxel level) (Table 2A and Fig.7). We also observed a large (64cm3) significant cluster (FWER p<0.05 cluster level) that included perigenual ACC, rostral bilateral ACC, left lateral OFC,frontaloperculum, DLPFC,posteriorACC and headof cau-dateaswellasrightdorso-medialPFCandfrontalpole(Table3A
andFig.7).
ThedirectedsearchwithinfiveVOIindicatedthatphysiological CPMwassignificantlypredictedbyearlycold-inducedresponses
Fig.7. Positiveresponsestoearly(outlinedwithwhiteframes)andnegativeresponsestosustainedcold-pain(greyshading)covariatingwith“physiologicalanalgesia”in posteriorinsula/SII.p<0.001uncorrectedmapsareshown.VOIsofdirectedsearchareoutlinedwithblackframes.Greatercold-inducedanalgesiaisrelatedtopositiveearly ornegativesustainedcold-painresponsesinACC,OFCandrostro-lateralPFC.
inallfiveVOI(medialandlateralOFC,ACC,leftrostralandright caudallateralPFC)(Table3A).
AsillustratedinFig.8formedialsubgenualACC,rostralACCand lateralOFCvoxels,BOLDresponseswerepositiveduringtheearly phaseofcoldconditioninginsubjectswhobecamehypoalgesicand hadreducedlaser-inducedactivationinleftposteriorinsula/SII,but negative(orlesspositive)inthosewhodevelopedhyperalgesia. Inthoseareastherewasaclearpositivecorrelationbetweenthe hemodynamicresponsesandphysiologicalCPM,asshowninFig.9 forthesubgenualACCVOI.
The correlationbetween the cerebralresponses during sus-tainedcoldconditioningandphysiologicalCPMweremorecomplex andvariablebetweenareas.Activationduringsustainedcold con-ditioning was positively related to physiological CPM in a few areasincludingcerebellum,coronaradiata,internalcapsula,dorsal putamenandinsula,andawhitematterclustercomprising ante-riorcoronaradiataandadjacentrostro-lateralPFC.(FWERp<0.05 clusterlevel).Thedirectedsearchconfirmeda singlesignificant cluster(FWERp<0.05clusterlevel)inrightrostro-lateralPFCVOI (Table3B).
By contrast, there was a negative correlation of sustained cold-inducedbrainactivitywithphysiologicalCPMinmostofthe prefrontalVOI(Table3C).Withthedirectedsearch,deactivation wassignificantlyrelatedtohypoalgesiaduringcoldconditioning inlateralOFC,ACC,leftrostro-lateralandrightcaudo-lateralPFC (Table3C).Atthewholebrainlevel,therewasasignificant clus-ter(6cm3,FWERp<0.05clusterlevel)includinglateralOFC,white matteranddorsolateralPFC(seeFig.7),aswellasthreesignificant clustersincerebellum.
3.3. Correlationswithanxietyandhabituation
Themean(trait)anxietyscoreinoursubjectswas36±1,range 26–51.Greatertraitanxietyscorescorrelatedoverallwith behav-ioralhyperalgesiaduringCPM(r=−0.46,p<0.05).
Accordingtotheretrospectivepainrating,therewasaslight decrease in cold pain during the application (from 6.0±0.5 to 5.1±0.4)andbetweenthefirstapplicationandthelastapplication (from6.7±0.4to4.5±0.4).CPMcorrelatedpositivelywith habit-uationofcoldpain,i.e.thedeclineofperceivedpaininthefootat theendoftheexperiment,(r=+0.49,p<0.05)andnegativelywith coldpainratingsattheendofthecompressapplication(r=−0.45, p<0.05).
Habituationofcoldpainratingscorrelatedwithcold-induced responsesinareasrelatedtophysiologicalCPM.Inparticular,like thelatter,habituationwasrelatedtoearlycold-inducedactivation inrostro-medialPFC[–66522],mOFC/sgACC[–1229–8]and cere-bellum[3–73–17](FWERp<0.05,clusterlevel)andothercluster inmOFCVOIconfirmedbydirectedsearch[632–14].Therewas,in addition,amedulla/caudalponscluster[6–40–35]wherenegative responsestosustainedcoldcorrelatedwithgreaterhabituationto cold(FWERp<0.05,clusterlevel).
4. Discussion
Theobjectiveofourstudywastobetterunderstandthe neu-ronalmechanismsof conditionedpainmodulation(CPM) using theheterotopicnoxious stimulationparadigm.Forthis purpose,
Fig.8.AveragetimeseriesofBOLDsignalsinprefrontalareaswhereearlycoldresponsescorrelatewith“physiologicalCPM”.VOIare6mmradiusspheresaroundthepeaks atmedialsubgenualanteriorcingulatecortex(sgACC[026−8]),anteriorcingulatecortex(ACC[94410])andlateralorbitofrontalcortex(lOFC[−3029−11])(Table3A). Eigenvariateswereextracted,normalizedtothemedianvalueofagiventimeseries,epochedandindividuallyaveragedforcoldleft)andlukewarm(right)conditionand thegroupaveragewasestimated.Thecriteriafordefining“physiologicalanalgesia”(dottedline)and“physiologicalhyperalgesia”(solidline)groupswereupperandlower quartilesofthephysiologicalanalgesiaparameterestimateextractedfromposteriorleftinsula/SIIVOI,anareawithbehaviorally-correlatedmodulationoflaser-induced neuronalresponses(seemethods2.5.2andresults3.2.5).
wesearchedwhetherindividualdifferencesbetweenconditioned modulationofbehavioralandcerebralresponsestothetestheat painwererelatedtothecerebralresponsesinducedbythecold conditioningstimulation.
4.1. Behavioralpainratings
Onaverage,overthe10consecutivetrials,therewasno signif-icantdecreaseinlaserpainratingsduringtheconditioningcold stimulation.FacilitatoryCPM(i.e.hyperalgesia)wasobservedin otherstudies,thoughoverallinasmallerproportionofsubjects. Inpre-operativepain-freepatients,facilitatoryCPMpredicts sub-sequentpost-surgerychronicpain[40]andhigheranalgesic con-sumption[41].ThelikelihoodtohavefacilitatoryCPMofaheattest stimulusbytourniquetischemicnoxiousconditioningwashigher insubjectswithlow5-HTTexpression[42].Inourstudy,only29% ofsubjectshadpainreduction,while29%hadnochangeand42% increasedlaserpain,clearlydemonstratinginter-individual differ-ences.ThiscontrastswithotherCPMstudies[15,16]andcouldhave severalnon-mutuallyexclusiveexplanations.
ThedegreeofCPMvariesbetweenstudiesanddependsonthe studydesign. For instance,CPM wasmarkedly greater(50%) in Pichéetal.[15]thaninSprengeretal.(20%)[16].Inourstudy,the conditioningcoldstimulationwasappliedrepeatedlycomparedto
a singlecounterstimulation inthetwopreviousstudies[15,16]. Individualdifferencesinhabituationtorepeatednoxiousstimuli maythusplayaroleinourparadigm,themoresothatwefound a positivecorrelationbetweenhabituation ofcold-inducedpain acrosssessionsand cold-inducedlaserpainreduction.Testpain producedbythetrainsofelectricshockslikeinPichéetal.’s pro-tocolmaybemoreamenabletoabehavioralCPMeffectthanpain inducedbyathermalstimuluswithsloweronsetandoffsetslopes usedinSprengeretal.’s[16]andinourstudy.Also,oursubjects ratedtheteststimulusaslesspainful(3.8/10NRS)comparedto thoseoftheabove-mentionedstudies.Thismayhavecontributed tothelackofconditionedhypoalgesiainsomesubjects,astheCPM effectcan beproportionaltothetest pain intensity[43]. How-ever,itisknownthatmoderatelypainfulteststimuli[15,44](about 40–50/100VAS),andevenonlyslightlypainfulones[31]are suffi-cienttoobtainreliableCPM.
Othermethodologicalissues arediscussedin Supplementary Material4.
Besides methodological aspects related to the experimental design, psychobehavioral factors might contribute to the large interindividualvariabilitywefoundforCPM.
Amongthem, apriori expectationis wellknowntostrongly correlate with drug-induced analgesia [45] and pain modula-tion[32,33,44].Inthecase ofpharmacologicalopioidanalgesia,
[33]. Stronger analgesia expectation and reduced stress during noxiouscoldconditioningwerelinkedtogreaterinhibitoryCPM [32].Althoughwekeptoursubjectsblindaboutthedirectionof expectedpainintensitychangesinducedbythecoldapplications, wecannotexcludethatexpectationplayedaroleinsomeofthem. Uncertainty about upcoming pain intensity also has a specific andpotenthyperalgesiceffect[46].Anxietyisknowntopartially mediatetheeffectofexpectationonthedirectionofCPM[44].Our findingthatanxietywasnegativelyrelatedwithbehavioralCPM supportsaroleofpsychologicalmodulators.
4.2. Cerebralresponsestotheconditioningcoldstimulation
Wefoundhighlysignificant(voxellevelFWERp<0.05) non-specificactivationsatonsetandoffsetofbothcoldandlukewarm stimulationsinmanyareasbelongingtotheso-called“painmatrix”. Themaximumofthisnon-specificresponsewasinleftSI,whichis consistentwithsomatosensoryresponsestothealternating sen-sorystimulationoftherightfoot.
By contrast, when early responses to cold were contrasted againstearlyresponsestolukewarm applicationtodetect pain-specificactivation,aclustercomprisingmainlybilateralprecuneus wassignificantly activatedbut not “pain matrix”areas suchas ACCandinsula.Theprecuneusisknowntorespondtomultimodal salientstimuli[47].
Wefoundnosignificantactivationinmostareasofthe“pain matrix”duringthesubsequentsustainedphaseofcoldapplication, withthenotableexceptionofposteriorinsula/SII.Thelatter activa-tionisconsistentwithacentralrolefortheoperculo-insularcortex inspecificpainprocessingandcodingofpainintensity[48–53]. Althoughourstudywasnotspecificallydesignedtoaddressthis question,itsresultsfavornonethelesstheconceptthatthe“pain matrix”ismostlya“saliencematrix”ofareasthatprocesssalient multimodalsensoryinputs[54]andthattheposteriorinsulamay bemorepain-specific[50,53].
Duringthesustainedphaseofcoldapplication,wefound deacti-vationinmedialOFC.Thisareaisknowntobeactivatedbyvaluation ofarewardoutcome[55]andwespeculatethatitsdeactivation couldbe related to the negative affective response due tothe sustainednoxiousstimulation.Theobservedclustersofsustained BOLDresponseinthewhitematterarediscussedinSupplementary Material5.
4.3. Cerebralresponsestothelaserteststimulusandtheir conditioningbycoldstimulation
Laserpain-inducedactivationsweresignificantinvariousareas of the “pain matrix” in line with a number of prior studies [47,56,57].Thisindicatesthattheintensityofthelaserstimuliwas sufficienttoinducecerebralresponsestypicallyreportedafter nox-iousstimulation.
Similartothebehaviorallaserpainratings(seeSection4.1),the laser-inducedBOLDresponsesdidnotdifferonaveragebetween cold and lukewarm conditioning.The inter-individual variation in CPM, however, that ranged from reduction to increase in painwascorrelatedwiththemodulationoftheBOLDresponse. Behavioralcold-inducedhypoalgesiawasassociatedwiththe sup-pression of laser-induced responses in posterior insula and SII contralateraltothecoldstimulation.Likesustainedcold-induced
Fig.9.RelationshipbetweenactivationofsgACCandbehavioralconditionedpain modulation.Eigenvariatesofbetaimagesforonlyearlycoldresponsewereextracted fromasphereof6mmradiusaroundthepeakofearlycoldvsearlywarmcontrast significantlycorrelatedto“physiologicalconditionedpainmodulation”(the eigen-variatesofthelaserwarmvslasercoldcontrastcorrelatedtobehavioralconditioned painmodulation)(Table3A).Thescatterpotisonlyforillustrationpurposes.
activation,thephysiologicalCPMthusinvolvedtheposteriorinsula, supportingagainitscriticalroleinpainaswellasCPMprocessing [50,53].
4.4. Correlationofcold-inducedcerebralresponseswith physiologicalCPM
In medial and lateral OFC, ACC, rostral and caudal lateral PFC,theearlycold-inducedBOLDresponsesweredirectlyrelated to subsequent CPM (see Figs. 8 and 9). These findings are in agreementwithotherstudies,where CPM wasassociated with activityevoked by thecold painstimulus inmPFC, lateralOFC [15,26]androstralACC[16].Bycontrast,BOLDactivationduring thelaterphaseofcoldstimulationwaslesspredictiveof physio-logicalCPM,asthecorrelationwassignificantonlyinrostro-lateral PFC.
Thecorrelationbetweenearlycold-inducedPFCresponsesand subsequentphysiological CPMcouldbe due tothefact thatthe conditioningstimulusisabletostrengthenconnectivitybetween corticalsourcesofdescendinganalgesiaandtheirtargetsin sub-cortical structures [58]. For instance, increased connectivity of subgenualACCwithmidbrainandamygdalawascorrelatedwith CPM[16].
Wealsofoundanunexpectedcorrelationbetween physiolog-icalCPManddeactivationinlateralOFC,ACC,lateralrostraland caudalPFCareasduringsustainedcoldapplication,whichwasnot reportedinothersstudies[15,16].Thismaybeduetothedifferent experimentaldesignofourstudy,butdifferencesinexpectation
orso-called“off-setanalgesia”couldbealternativeexplanations. Inamodelofvisceralpain,expectation-mediatedplacebo analge-siawasassociatedwithfMRIdeactivationinthedorsolateralPFC [59].NegativeBOLDresponsesinventro-medialPFC/OFCwerealso recordedduringlargereductionsofpainratinginducedbyaslight decreaseofa noxiousstimulation,i.e. off-setanalgesia[8].This paradigmcouldberelevantforourstudywheretherewas habit-uationofcoldpainratingsbetweenthe1standlastapplications, andhenceadecreaseinperceivedpain.
4.5. Correlationswithcoldpainhabituation
Habituationofcoldpainperceptioncorrelatedpositivelywith behavioral CPM, with early cold-induced lateral OFC activation thatisalsorelatedtophysiologicalCPM,andwithsustained cold-induceddeactivationinpons/cerebellum.Habituationofpainover severaldayswasfoundassociatedwithsubgenualACCactivation [60].Habituationthuslikelyinvolvescentralneuronalnetworks thatitsharesatleastpartiallywithCPM.Diffusenoxiousinhibitory control,non-noxiousinhibitorycontrolandhabituationareallpart ofendogenousanalgesiaandthoughttoberelatedtoeachother [43].
Toconclude,ourstudyconfirms that sustainednoxiouscold stimulationchieflyactivatesposteriorinsula/SII.Activationinother areasoftheso-called“painmatrix”appearstobemerely deter-minedbysaliencyandnoveltyratherthanbythenoxiouscharacter ofthestimulus.Wefindgreatinter-individualdifferencesinCMP thatrangesfromdecreasestoincreasesofthetestlaser-heatpain. LargerprefrontalpositiveBOLDresponsestoearlycold stimula-tionseemtopredictsubsequentconditionedanalgesia.Bycontrast, inadjacentprefrontalcorticesconditionedanalgesiaisassociated withdeactivationduringthesustainedconditioningcold stimula-tion.WeshowthatCPMisnegativelycorrelatedwithtraitanxiety, butpositivelywithhabituationofcoldpain.Theindividual differ-encesinprefrontalcortexactivityandCPMneedtobeconsidered infuturestudiesofdiseasedsubjects,includingmigraineurs. Acknowledgements
Thisstudywassupportedbytheresearchconvention3.4.650.09 fromtheNationalFundforScientificResearch–BelgiumtoJ.S.,and byaresearchgrantfromtheCHUofClermont-Ferrand–Franceto R.D.andJ.S.WearegratefultoDrsAndréLuxen,EricSalmonand PierreMaquetfororganizational andtoChristianDegueldrefor technicalassistance.
Theauthorsdeclarenoconflictsofinterest. AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,athttp://dx.doi.org/10.1016/j.bbr.2014.11.028. References
[1]MayerDJ,LiebeskindJC.Painreductionbyfocalelectricalstimulationofthe brain:ananatomicalandbehavioralanalysis.BrainRes1974;68:73–93.
[2]LeBarsD,ChitourD,KrausE,ClotAM,DickensonAH,BessonJM.Theeffectof systemicmorphineupondiffusenoxiousinhibitorycontrols(DNIC)intherat: evidenceforaliftingofcertaindescendinginhibitorycontrolsofdorsalhorn convergentneurones.BrainRes1981;215:257–74.
[3]GearRW,AleyKO,LevineJD.Pain-inducedanalgesiamediatedbymesolimbic rewardcircuits.JNeurosci1999;19:7175–81.
[4]LevineJD,GordonNC,FieldsHL.Themechanismofplaceboanalgesia.Lancet 1978;2:654–7.
[5]ValetM,SprengerT,BoeckerH,WillochF,RummenyE,ConradB,etal. Dis-tractionmodulatesconnectivityofthecingulo-frontalcortexandthemidbrain duringpain—anfMRIanalysis.Pain2004;109:399–408.
[6]FreundW,KlugR,WeberF,StuberG,SchmitzB,WunderlichAP.Perception andsuppressionofthermallyinducedpain:afMRIstudy.SomatosensMotRes 2009;26:1–10.
[7]YilmazP,DiersM,DienerS,RanceM,WessaM,FlorH.Braincorrelatesof stress-inducedanalgesia.Pain2010;151:522–9.
[8]YelleMD,OshiroY,KraftRA,CoghillRC.Temporalfilteringofnociceptive informationbydynamicactivationofendogenouspainmodulatorysystems. JNeurosci2009;29:10264–71.
[9]LeknesS,BernaC,LeeMC,SnyderGD,BieleG,TraceyI.Theimportanceof context:whenrelativereliefrenderspainpleasant.Pain2013;154:402–10.
[10]Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induceddiffusenoxiousinhibitorycontrol(DNIC)-likeeffectinhumans.Pain 2009;144:16–9.
[11]YarnitskyD,Arendt-NielsenL,BouhassiraD,EdwardsRR,FillingimRB, Gra-notM,etal.Recommendationsonterminologyandpracticeofpsychophysical DNICtesting.EurJPain2010;14:12.
[12]CarstensE,YokotaT,ZimmermannM.Inhibitionofspinalneuronalresponses tonoxiousskinheatingbystimulationofmesencephalicperiaqueductalgray inthecat.JNeurophysiol1979;42:558–68.
[13]Villanueva L, Le Bars D. The activation of bulbo-spinal controls by peripheralnociceptiveinputs:diffusenoxiousinhibitorycontrols.BiolRes 1995;28:113–25.
[14]SlukaKA,DeaconM,StibalA,StrisselS,TerpstraA.Spinalblockadeofopioid receptorspreventstheanalgesiaproducedbyTENSinarthriticrats.JPharmacol ExpTher1999;289:840–6.
[15]PicheM,ArsenaultM,RainvilleP.Cerebralandcerebrospinalprocesses under-lyingcounterirritationanalgesia.JNeurosci2009;29:14236–46.
[16]SprengerC,BingelU,BuchelC.Treatingpainwithpain:supraspinal mecha-nismsofendogenousanalgesiaelicitedbyheterotopicnoxiousconditioning stimulation.Pain2011;152:428–39.
[17]vanWijkG,VeldhuijzenDS.Perspectiveondiffusenoxiousinhibitorycontrols asamodelofendogenouspainmodulationinclinicalpainsyndromes.JPain 2010;11:408–19.
[18]PielstickerA,HaagG,ZaudigM,LautenbacherS.Impairmentofpaininhibition inchronictension-typeheadache.Pain2005;118:215–23.
[19]SandriniG,RossiP,MilanovI,SerraoM,CecchiniAP,NappiG.Abnormal modu-latoryinfluenceofdiffusenoxiousinhibitorycontrolsinmigraineandchronic tension-typeheadachepatients.Cephalalgia2006;26:782–9.
[20]de Tommaso M, Difruscolo O, Sardaro M,Libro G, Pecoraro C, Serpino C, et al. Effects of remote cutaneous pain on trigeminal laser-evoked potentials in migraine patients. J Headache Pain 2007;8: 167–74.
[21]StaudR,RobinsonME,VierckJrCJ,PriceDD.Diffusenoxiousinhibitorycontrols (DNIC)attenuatetemporalsummationofsecondpaininnormalmalesbutnot innormalfemalesorfibromyalgiapatients.Pain2003;101:167–74.
[22]NormandE,PotvinS,GaumondI,CloutierG,CorbinJF,MarchandS.Pain inhi-bitionisdeficientinchronicwidespreadpainbutnormalinmajordepressive disorder.JClinPsychiatry2010;72:219–24.
[23]Wilder-SmithCH,SchindlerD,LovbladK,RedmondSM,NirkkoA.Brain func-tionalmagneticresonanceimagingofrectalpainandactivationofendogenous inhibitorymechanismsinirritablebowelsyndromepatientsubgroupsand healthycontrols.Gut2004;53:1595–601.
[24]KosekE,OrdebergG.Lackofpressurepainmodulationbyheterotopicnoxious conditioningstimulationinpatientswithpainfulosteoarthritisbefore,butnot following,surgicalpainrelief.Pain2000;88:69–78.
[25]Wilder-SmithOH,SchreyerT,SchefferGJ,Arendt-NielsenL.Patientswith chronicpainafterabdominalsurgeryshowlesspreoperativeendogenouspain inhibitionandmorepostoperativehyperalgesia:apilotstudy.JPainPalliatCare Pharmacother2010;24:119–28.
[26]MoontR,CrispelY,LevR,PudD,YarnitskyD.Temporalchangesincortical activationduringconditionedpainmodulation(CPM),aLORETAstudy.Pain 2011;152:1469–77.
[27]SongGH,VenkatramanV,HoKY,CheeMW,YeohKG,Wilder-SmithCH.Cortical effectsofanticipationandendogenousmodulationofvisceralpainassessedby functionalbrainMRIinirritablebowelsyndromepatientsandhealthycontrols. Pain2006;126:79–90.
[28]Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responsestopain.Areviewandmeta-analysis.NeurophysiolClin2000;30: 263–88.
[29]HarperRM,MaceyPM,HendersonLA,WooMA,MaceyKE,FrysingerRC,etal. fMRIresponsestocoldpressorchallengesincontrolandobstructivesleep apneasubjects.JApplPhysiol2003;94:1583–95.
[30]MaceyPM, MaceyKE, Woo MA,Keens TG, HarperRM. Aberrantneural responsestocoldpressorchallengesincongenitalcentralhypoventilation syn-drome.PediatrRes2005;57:500–9.
[31]PlaghkiL,DelisleD,GodfraindJM.Heterotopicnociceptiveconditioningstimuli andmentaltaskmodulatedifferentlytheperceptionandphysiological corre-latesofshortCO2laserstimuli.Pain1994;57:181–92.
[32]Bjorkedal E, Flaten MA. Expectations of increased and decreased pain explaintheeffectofconditionedpainmodulation infemales.J PainRes 2012;5:289–300.
[33]GoffauxP,RedmondWJ,RainvilleP,MarchandS.Descendinganalgesia—when thespineechoeswhatthebrainexpects.Pain2007;130:137–43.
[34]SpielbergerCD,GoruchRL,LushenePR,VaggPR,JacobsGA.Manualforthe state-traitanxietyinventory(formY).PaloAlto,CA:ConsultingPsychologists Press;1983,36p.
2002;16:217–40.
[39]AshburnerJ,FristonKJ.Unifiedsegmentation.Neuroimage2005;26:839–51.
[40]YarnitskyD,CrispelY,EisenbergE,GranovskyY,Ben-NunA,SprecherE,etal. Predictionofchronicpost-operativepain:pre-operativeDNICtestingidentifies patientsatrisk.Pain2008;138:22–8,.Epub2008Jan8.
[41]GrosenK,VaseL,PilegaardHK,Pfeiffer-JensenM,DrewesAM.Conditionedpain modulationandsituationalpaincatastrophizingaspreoperativepredictorsof painfollowingchestwallsurgery:aprospectiveobservationalcohortstudy. PLoSOne2014;9:2014.
[42]LindstedtF,BerrebiJ,GreayerE,LonsdorfTB,SchallingM,IngvarM,etal. Con-ditionedpainmodulationisassociatedwithcommonpolymorphismsinthe serotonintransportergene.PLoSOne2011;6:0018252.
[43]TreisterR,EisenbergE,GershonE,HaddadM,PudD.Factorsaffecting—and rela-tionshipsbetween—differentmodesofendogenouspainmodulationinhealthy volunteers.EurJPain2010;14:608–14.
[44]CormierS,PicheM,RainvilleP.Expectationsmodulateheterotopicnoxious counter-stimulationanalgesia.JPain2013;14:114–25.
[45]BingelU,WanigasekeraV,WiechK,NiMhuircheartaighR,LeeMC,Ploner M, etal. Theeffect oftreatment expectation ondrug efficacy: imaging the analgesic benefit ofthe opioid remifentanil. SciTransl Med 2011;3, 70ra14.
[46]YoshidaW,SeymourB,KoltzenburgM,DolanRJ.Uncertaintyincreasespain: evidenceforanovelmechanismofpainmodulationinvolvingthe periaque-ductalgray.JNeurosci2013;33:5638–46.
[47]Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigationofthefunctionalsignificanceofthepainmatrix.Neuroimage 2011;54:2237–49.
[52]FairhurstM,FairhurstK,BernaC,TraceyI.AnfMRIstudyexploringtheoverlap anddifferencesbetweenneuralrepresentationsofphysicalandrecalledpain. PLoSOne2012;7:e48711.
[53]PomaresFB,FaillenotI,BarralFG,PeyronR.The‘where’andthe‘when’ofthe BOLDresponsetopainintheinsularcortex.Discussiononamplitudesand latencies.Neuroimage2013;64:466–75.
[54]IannettiGD,MourauxA.Fromtheneuromatrixtothepainmatrix(andback). ExpBrainRes2010;205:1–12.
[55]O’DohertyJ,KringelbachML,RollsET,HornakJ,AndrewsC.Abstractrewardand punishmentrepresentationsinthehumanorbitofrontalcortex.NatNeurosci 2001;4:95–102.
[56]Mobascher A,BrinkmeyerJ, WarbrickT,MussoF, WittsackHJ,Stoermer R, et al.Fluctuations inelectrodermal activityreveal variationsinsingle trialbrainresponsestopainfullaserstimuli—afMRI/EEGstudy.Neuroimage 2009;44:1081–92.
[57]WatsonA,El-DeredyW,IannettiGD,LloydD,TraceyI,VogtBA,etal.Placebo conditioningandplaceboanalgesiamodulateacommonbrainnetworkduring painanticipationandperception.Pain2009;145:24–30.
[58]KongJ,JensenK,LoiotileR,CheethamA,WeyHY,TanY,etal.Functional con-nectivityofthefrontoparietalnetworkpredictscognitivemodulationofpain. Pain2013;154:459–67.
[59]ElsenbruchS,KotsisV,BensonS,RosenbergerC,ReidickD,SchedlowskiM,etal. Neuralmechanismsmediatingtheeffectsofexpectationinvisceralplacebo analgesia:anfMRIstudyinhealthyplaceborespondersandnonresponders. Pain2012;153:382–90.
[60]BingelU,SchoellE,HerkenW,BuchelC,MayA.Habituationtopainful stimu-lationinvolvestheantinociceptivesystem.Pain2007;131:21–30.