The bromine and chlorine isotopic composition of the mantle as revealed by
deep geothermal fluids
Daniele L. PINTI1, Orfan SHOUAKAR-STASH2, M. Clara CASTRO3, Aida LOPEZ-HERNÁNDEZ4, Chris M. HALL3, Océane ROCHER1,5, Tomo SHIBATA6, Miguel
RAMÍREZ-MONTES7
1GEOTOP Research Center on the dynamics of the Earth System, Université du Québec à Montréal, H3X 3Y7 QC, Canada
2Isotope Tracer Technologies Inc., Waterloo, ON, Canada
3Department of Earth and Environmental Sciences, University of Michigan, Ann Arbor, MI, USA 4Facultad de Ingeniería Civil, Universidad Michoacána de San Nicolas de Hidalgo (UMSNH), Morelia, Mich., México
5Ecole Nationale Supérieure de Géologie, 2 Rue du doyen Marcel Roubault, 54518 Vandœuvre-lès-Nancy, France
6Institute for Geothermal Sciences, Graduate School of Science, Kyoto University, Beppu, Oita 874-0903, Japan
7Gerencia de Proyectos Geotermoeléctricos, Comisión Federal de Electricidad, Morelia, Mich., México
* Corresponding author: pinti.daniele@uqam.ca
Keywords: bromine isotopes; chlorine isotopes; helium isotopes; geothermal fluids; mantle; halogen cycling. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
ABSTRACT. Chlorine (Cl) and bromine (Br) are rare elements when considering the whole
Earth. However, being highly volatile elements, their delivery and retention processes during planetary accretion and chemical differentiation provide important clues about the formation of the Earth. Variations in Cl and Br isotopic systems (37Cl/35Cl or 37Cl and 81Br/79Br or 81Br) among terrestrial reservoirs could trace these processes. While the Br isotopic value of the mantle remains entirely unknown, the measurement of mantle Cl isotopic values is a controversial subject, with measured 37Cl values ranging from -3 to 0‰ in mid-ocean-ridge basalts (MORBs) and from -2 to +3‰ in oceanic island basalts (OIBs). Here, we report newly-determined 81Br and 37Cl values, together with noble gas 3He/4He (R) ratios, measured in geothermal fluids from production wells of three Mexican fields: Cerro Prieto, Las Tres Vírgenes, and Los Azufres. Relationships between 3He/4He ratios and both 37Cl and 81Br suggest that geothermal fluid volatiles have three distinct sources: 1) a local crustal source, enriched in radiogenic 4He (R = 1.7-1.9Ra, where Ra is the atmospheric 3He/4He ratio), and halogens from brines with 37Cl and 81Br of +0.1 and +0.3‰ respectively; 2) the mantle wedge, with 3He/4He ratios of 6-6.5Ra, typical of arc volcanism, and 37Cl and 81Br of -0.4 and -1.0‰ respectively, typical (for Cl) of fluids derived from the dehydration of serpentinite in the subducting slab; and 3) a mantle source, with 3He/4He ratios of 7.7-8.2Ra, typical of MORBs, and 37Cl and 81Br of +0.9 and +0.7‰ respectively. These results suggest that the primitive mantle Cl isotopic composition was positive – possibly +3‰, as measured in some OIBs – and inherited during the Moon forming impact. The progressive subduction of isotopically lighter halogens over the last 2-3 Ga could have progressively lowered this initial value to those currently measured in the depleted mantle beneath Mexico. It is speculated that the different isotopic values measured in mantle rocks and fluids could reflect the heterogeneous regassing of subducted halogens and the inefficient 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46
homogenization of recycled material in the MORB source, as suggested in other studies by the heterogeneous isotopic compositions of the heavier Ar and Xe of the convective mantle. 47
48 49 50
1. INTRODUCTION
Halogen elements, Cl and Br, are minor within the Solar system and are relatively rare when considering the whole Earth (Eggenkamp, 2014). They are highly volatile and reactive elements, displaying a preference for fluids (Eggenkamp, 2014; Clay et al., 2017). This is why they are mainly concentrated in the Earth’s surface reservoirs – the oceans (Cl = 19353 ppm and Br = 67 ppm) and evaporites (Cl = 607,000 ppm and Br = 151 ppm) (Eggenkamp, 2014).
Oceanic Cl and Br are sourced from the depleted mantle (Schilling et al., 1978), which is well degassed (up to 90%; Burgess et al., 2002) of its halogen contents (Cl = 16 ppm and Br < 0.05 ppm in the present-day depleted mantle; Eggenkamp, 2014).
Because of halogen’s volatility and incompatibility with silicate Earth, their delivery and retention processes during planetary accretion and chemical differentiation provide important clues about the Earth and the formation of its surface reservoirs. Clay et al. (2017) have measured Cl and Br abundances in chondrites, and found that they are much lower (from 6 to 9 times) than previous estimates (e.g., Donough, 2003). However, comparison of the chondritic halogen inventory with that of the bulk silicate Earth (BSE) and of other volatile species suggests that halogens have not undergone the extreme early loss observed for other mantle volatiles, such as noble gases (e.g., Marty, 2012). The hydrophilic nature of halogens could have played a role in preserving them in surface aqueous phases during early accretionary events (Clay et al., 2017). Furthermore, the Br/Cl ratios measured in chondrites (Br/Cl = 0.3-4.7 x 10-3; Clay et al., 2017) are within the range of those measured in the mantle sourcing mid-ocean-ridge basalts (MORBs; Br/Cl = 0.9-10 x 10-3) and those estimated for the BSE (Br/Cl =2.9-4 x 10-3; e.g., Donough, 2003). This observation implies that the mechanism of Cl and Br delivery to the Earth, and their degassing to the surface, occurred with negligible elemental fractionation (Clay et al., 2017). 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73
These few observations highlight the importance of precisely measuring halogen contents in terrestrial reservoirs, and particularly of obtaining the precise isotopic composition of Cl and Br – the only two halogens with stable isotopes – in order to univocally constrain any model controlling halogen (and other volatile) delivery to Earth.
37Cl values have been measured in numerous magmatic rocks, yet general consensus on the Cl isotopic composition of the depleted mantle is still lacking. Sharp et al. (2007) measured chlorine isotopes in carbonaceous chondrites, MORBs, sub-continental mantle rocks, and crustal rocks from the Archean to present, and estimated depleted mantle 37Cl to be close to -0.2±0.5‰ vs. Standard Mean Oceanic Chloride (SMOC), similar to the modern seawater value (37Cl/35Cl = 0.31977; Shields et al., 1963; SMOC = 0‰ by definition; Eggenkamp and Coleman, 2000). They concluded that Cl originated from an isotopically-homogenous nebular reservoir, and that there was no isotopic fractionation during the accretion and differentiation of the Earth. The nearly identical 37Cl values of the depleted mantle and the ocean indicate that mantle degassing did not produce any resolvable isotopic fractionation, as observed during volcanic degassing (e.g., Pyle and Mather, 2009; Barnes et al., 2014). These results support the hypothesis of Clay et al. (2017), of little elemental and isotopic fractionation during halogen accretion to the Earth and degassing from the mantle to the surface.
Bonifacie et al. (2008b) carefully measured 37Cl in N-MORB and E-MORB glasses from several oceanic rides, together with Cl and K contents. They obtained an relationship between 37Cl and the invesre of the Cl content, which suggested that high-37Cl, Cl-rich basalts are contaminated by seawater, whereas low-37Cl, Cl-poor basalts approach the composition of the depleted mantle, with an extrapolated 37Cl value of -1.6‰. The isotopic difference between the surface of the Earth (0‰) and the depleted mantle (-1.6‰) was attributed to either net Cl 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96
isotopic fractionation associated with the removal of Cl from the mantle and its return by
subduction, and/or the addition of a 37Cl-enriched Late Veneer (Bonifacie et al., 2008b). Analyses of Cl isotopes in MORBs from the Lau Basin suggested that the depleted mantle reservoir could have an even lighter composition, of ca. -3‰ (Layne et al., 2009). In contrast, John et al. (2010) measured the 37Cl in oceanic island basalts (OIBs), finding a more heterogeneous mantle reservoir, with 37Cl ranging from -1.6 to +2.9‰. John et al. (2010) compared the high 37Cl values measured in OIBs (+2.9‰) with those measured in subducted marine metasediments (37Cl from -2 to +2‰). They hypothesized that subducting sediments may have developed high δ37Cl values by expelling 37Cl-depleted pore fluids during serpentinite dehydration (e.g.,
Bonifacie et al., 2008a), thus accounting for the positive δ37Cl values recorded in OIB lavas. Early on, Br isotopes were not analyzed in mantle samples, because of their very low concentrations in this reservoir (i.e., the Cl/Br ratio in N-MORB is 2500; e.g., Jambon et al., 1995), leading to technical difficulties, which have been overcome in the last two decades (Eggenkamp and Coleman, 2000; Shouakar-Stash et al., 2005b; Louvat et al., 2016). At present, 81Br measurements are possible in fluids (Frape et al., 2007; Shouakar-Stash et al., 2007; Shouakar-Stash, 2008; Stotler et al., 2010; Bagheri et al., 2014), because of the large amount of material available to extract and concentrate bromine prior to isotopic analysis.
Here, we present 81Br and 37Cl values together with helium isotopic ratios (R/Ra with R = 3He/4He, normalized to atmospheric helium ratio, Ra = 1.384 x 10-6; ), measured in
high-enthalpy brines from deep geothermal production wells from the Mexican geothermal fields of Cerro Prieto, Los Azufres, and Las Tres Vírgenes (Fig. 1). Geothermal brines are a mixture of mantle fluids, sedimentary porewater, and meteoric waters (e.g., Giggenbach, 1992; Hedenquist 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118
and Lowenstern, 1994; Yardley and Bodnar, 2014), and volatiles from these three terrestrial reservoirs are expected to be found (e.g., Mazor and Truesdell, 1984; Pinti et al., 2019a).
2. GEOLOGICAL BACKGROUND
Geothermal fluids analyzed for their Cl and Br isotopic contents are from three Mexican geothermal fields, which are known to be high-temperature (> 200˚C) convection-dominated geothermal reservoirs (Moeck and Beardsmore, 2014). These fields invariably lie on tectonic plate margins, on regions of active volcanism and plutonism, or extensional tectonic areas characterized by crustal thinning and elevated heat flow (Moeck and Beardsmore, 2014).
The Cerro Prieto Geothermal Field (CPGF) is located in Baja California (Fig. 1). It is the largest high-enthalpy (> 280˚C) liquid-dominated geothermal system in Mexico. Currently, 147 operating wells drilled at depths varying between 1,000 and 4,400 meters below surface extract 34.6 million metric tons of steam per year (Gutiérrez-Negrín, 2015). The reservoir is located in a pull-apart basin formed by the active strike-slip Imperial Fault in the northeast and the Cerro Prieto Fault in the southwest of the field, both belonging to the San Andreas Fault System (Suárez-Vidal et al., 2008). The heat source is gabbros intruded as a stress response of the extensional crustal thinning (Elders et al., 1984). A 2,400 m-thick sequence of Plio-Pleistocene deltaic sedimentary rocks (Colorado River sandstones interbedded with gray shales) hosts the geothermal reservoir (Lira-Herrera, 2005), capped by shales and mudstones. The unconsolidated quaternary clastic sediments of the Colorado River lie at the top of the stratigraphic sequence (Lira-Herrera, 2005), in which porewater likely constitutes the natural recharge of the field (Pinti et al., 2019a). Reservoir temperature ranges from 250 to 310˚C. Water is of sodium chloride type with neutral to alkaline pH (Gutiérrez-Negrín, 2015). An inverse relationship between the Br/Cl 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141
Prieto geothermal fluids derive from the mantle and a seawater component modified by halite dissolution.
The Los Azufres Geothermal Field (LAGF) is located in the Trans-Mexican Volcanic Belt (TMVB; Fig. 1), in the central Michoacán State. The field is located in the southern portion of a silicic volcanic complex filled with 2,700 meter-thick Upper Miocene to Pliocene (23-7 Ma) basalt-andesite ignimbrite sequences intercalated with lavas, called the Mil Cumbres andesite unit (Pérez et al., 2010) (Fig. 2). This unit constitutes the geothermal reservoir, with fluids of sodium chloride type, pH between 5.8 and 7.2 (Birkle et al., 2001), and temperatures between 240 and 320˚C. It is capped by a succession of rhyo-dacitic and basaltic units: the 600 m-thick andesitic lavas and basaltic andesite sequences of the Marítaro-Tejamaniles unit, the Las Humaredas-San Andrés dacite unit (1.22-0.33 Ma), and the Aqua Fria-La Yerbabuena rhyolite unit (1.03-0.02 Ma). The field is divided geographically into two zones; the Northern Production Zone (NPZ), where reservoir conditions are in the compressed liquid region, and the Southern Production Zone (SPZ), which has different systems depending on depth, varying from vapor-dominated, to liquid-dominated, and to compressed-liquid regions as depth becomes shallower
(Torres-Rodríguez et al., 2005). The main heat source is likely MORB-like parental magmas, as suggested by helium (R/Ra up to 7.93±0.09) and strontium (87Sr/86Sr lower than
0.70375±0.00005) isotopic signatures of the fluids (Wen et al., 2018). This suggests the presence of pure lithospheric mantle melts beneath the field, a hypothesis supported by the carbon isotopic signature of the fluids (average 13C = -7.58±0.59; Richard et al., 2019), which is within that expected for the depleted mantle (13C -6±2‰; e.g., Javoy et al., 1986). The presence of such melts in a region dominated by subduction is explained by the particular geometry of the Cocos 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164
plate that plunges almost vertically in the middle of the TMVB, allowing deep melts to rise, underplating the North America plate (Richard et al., 2019).
The Las Tres Vírgenes Geothermal Field (LTVGF) is located in Baja California Sur (Fig. 1), in a NW-SE-oriented Plio-Quaternary rift, called the Santa Rosalía Basin (López-Hernández et al., 1995). This basin constitutes the western limit of the deformation zone related to the Gulf of California opening, which created several young oceanic basins interconnected by transform faults (Fig. 1) (Arango-Galván et al., 2015). During the Miocene, this area was the subduction zone of the Farallon plate under the American plate (Comondù arc in Fig. 1; Ferrari et al., 2012). The thermal activity of the field is concentrated at the border of the Las Tres Vírgenes volcano complex (0.44 Ma- 30 ka) (López-Hernández et al., 1995). The reservoir is low-permeability Upper Cretaceous granodiorites capped by 750 m of Upper Oligocene to Middle Miocene volcano-sedimentary sandstones and andesites (Santa Lucía Formation) of the Comondú Group, (López-Hernández et al., 1995). Overlying the Comondú Group is the Late Miocene Esperanza Formation, initially described as tholeiitic basalts, but now considered to be the product of subduction, similar to adakites (Ferrari et al., 2012). The shallow regional aquifer recharges the reservoir, which has temperatures ranging from 250 to 275˚C (Tello-López and
Torres-Rodríguez, 2015). The water fraction is of sodium chloride to bicarbonate-sulfate type, with a neutral pH (Barragán et al., 2009). Halogen ratios suggest that geothermal fluids are seawater modified by halite dissolution. The helium isotopic signature of the mantle fluid component (R/Ra = 6.19) suggests either a sub-continental mantle source (e.g., Gautheron and Moreira, 2002) or a depleted mantle source contaminated by older sediments derived from the Farallon subducting plate (Pinti et al., 2019b). This last hypothesis is in agreement with the sediment-like carbon isotopic signatures found in LTV (Richard et al., 2019) and recent chemical anomalies 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187
2019) from several locations in Baja California, which suggest that parental magmas under this region might be generated by the partial melting of the remains of the subducted Farallon plate.
3. SAMPLE SELECTION AND ANALYTICAL METHODS
Chlorine and bromine elemental ratios and 37Cl and 81Br values were obtained from water samples collected from the geothermal wells. Fumarole and hot spring fluids, which underwent phase segregation and boiling during their ascent to the surface (Arnórsson et al., 2007), were avoided. Sharp et al. (2010a) have demonstrated that, in high-temperature fumaroles, 37Cl is strongly partitioned into the vapor phase during boiling, creating very high 37Cl values, of up to +12‰. This is best explained by a distillation process, in which 35Cl is preferentially incorporated as an aqueous chloride species in the condensate, leaving the HCl(g) in the boiled phase with increasing 37Cl values (Sharp et al., 2010a). Water samples for Cl and Br isotope analyses were collected at the wells’ weirboxes (i.e., the fabricated channel into which a weir plate is installed; located at the exit of the well silencer), using 1L Nalgene bottles.
Samples for noble gas analyses were collected in standard refrigeration-grade 3/8” copper type K tubes, sealed by stainless steel pinch-off clamps. Gases were collected at the wellheads just after the steam/water separator by fixing the copper tube to a small stool, aligned with one of the output valves of the steam conduit. A single copper tube was extended from the sampler to the NPT-type male connector screwed onto the steam conduit valve. The clamps were closed using electric drills to reduce the risk of air contamination (e.g., Wen et al., 2018). Because noble gases are incondensable, and nearly completely partitioned into the steam phase and thus
concentration, elemental and isotopic ratios reflect those of the geothermal fluid at depth. 189 190 191 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210
The Cl and Br data reported in this study are all original, with the exception of data from Las Tres Vírgenes, which were previously published in Pinti et al. (2019b) (Table 1). Helium isotopic data, with the exception of a few duplicates obtained in a later survey in 2018 (Table 1), were also published elsewhere (Pinti et al., 2013; Wen et al., 2018; Pinti et al., 2019a; 2019b). We took particular care to avoid samples that could have been affected by reinjection. Re-injection consists of injecting a mixture of fluids collected from several wells back into the reservoir by gravity to preserve the pressure of the reservoir. This mixture is full of atmospheric volatiles, contaminating the helium isotopic ratio and shifting its pristine value toward the air composition (R/Ra = 1) (Pinti et al., 2017; Wen et al., 2018). The Cl and Br isotopic
compositions could be affected by evaporation processes and isotopic fractionation. The Cl and Br isotopic compositions were measured in four re-injected fluids at wells AZ-3, AZ-7, AZ-8, and AZ-61, and resulted in 37Cl and 81Br values ranging from +0.3 to +1‰ and +0.4 to +0.6‰ respectively. These values are within the ranges of those measured in the production wells of Los Azufres, or +0.3‰ higher for 37Cl, suggesting that little Cl or Br isotopic fractionation is
produced during used brine collection and its re-injection into the reservoir. R/Ra values, 37Cl, and 81Br were measured from well AZ-2A, which is well known to be 90% contaminated by re-injected fluids (Pinti et al., 2013). The R/Ra value was 2.72, indicating a large addition of atmospheric helium (Wen et al., 2018), but 37Cl and 81Br values were 0.05±0.08‰ and
0.00±0.13‰, within the range of values measured in production wells. A shift of +0.3‰ between samples measured in 2016 and in 2018 was observed at Las Tres Virgenes (not reported here), which is suspected to indicate an incipient re-injection effect in the field (Pinti et al., 2019b). The possible effects of different geothermal reservoir processes (re-injection, gas-water partition, 211 212 213 214 215 216 217 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232
adiabatic boiling, etc.) on Cl and Br isotopes will be discussed further in the Discussion section, and are illustrated schematically in Fig. 6.
Chlorine and bromine stable isotope analyses were conducted on methyl chloride (CH3Cl) gas and methyl bromide (CH3Br) gas respectively, after converting chloride and bromide ions in solution to CH3Cl and CH3Br gases through a multi-step procedure (Shouakar-Stash et al., 2005a; 2005b). The ratios of Cl stable isotopes (37Cl/35Cl) and Br stable isotopes (81Br/79Br) were determined by continuous-flow isotope ratio mass spectrometry (IRMS)(Shouakar-Stash et al., 2005a; 2005b). A Micromass® IsoPrime was used to measure 37Cl, and an Agilent 6890 gas chromatograph (GC) equipped with a CTC Analytics CombiPAL autosampler was attached to the IRMS for CH3Cl and CH3Br separation. Sample and standard measurements consisted of
measuring the separated CH3Cl and CH3Br against a set of reference gas (CH3Cl, CH3Br) pulses. Reference gases were measured 6-8 times and the isotopic ratio of the sample or standard peak was determined against the average of the reference gas readings. Each sample was measured 2-4 times. All results were corrected and reported against SMOC and Standard Mean Oceanic
Bromide (SMOB). A calibrated internal standard was used during every run. The analytical precision on the Cl and Br standards is ± 0.2‰ (2).
The noble gas analyses were carried out at the Noble Gas Laboratory of the University of Michigan. Gas samples, connected to a stainless-steel extraction and purification line, were dried on a molecular sieve trap and active gases were removed using three Ti sponge getters at 600°C for three minutes each. Helium was quantitatively extracted using a computer-controlled cryo-separator at a temperature of 49 K and sequentially allowed to enter the Thermo Scientific® Helix SFT mass spectrometer. 4He was measured using a Faraday detector, and 3He was measured using an electron multiplier in ion counting mode. Quantitative analyses were obtained by 233 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253 254 255
calibrating the mass spectrometer with a known aliquot of standard air. The standard error on helium concentration was 1.5%. A detailed description of the analytical procedures can be found in Wen et al. (2018) and Pinti et al. (2019a).
4.RESULTS
Table 1 reports the 3He/4He ratios (R) normalized to that of the atmosphere (Ra = 1.384 x 10-6), or R/Ra values. These values were corrected for the air component, following the classic method of Craig et al. (1978):
Rc/ Ra=[(R/ Ra)−r ]/(1−r ) (1) He ❑ 4 ¿ Ne ¿ ¿❑ 20 ¿ He ❑ 4 ¿ Ne ¿ ¿20❑ ¿ ¿ ¿ r =¿ (2)
where Rc/Ra represents R/Ra corrected for the air component (Table 1); (R/Ra)meas is the measured value; and (4He/20Ne)AIR and (4He/20Ne)meas represent the isotopic ratios of the air component and the collected sample, respectively. An air component could either be added accidentally during sampling or represent meteoric recharge (i.e., the atmospheric helium dissolved at solubility equilibrium in meteoric water at the recharge of the field (Air Saturated Water (ASW), calculated for a given temperature)). In the case of contamination during
sampling, (4He/20Ne)AIR is equal to 0.3185 (Ozima and Podosek, 1983), and in the case of ASW, (4He/20Ne)AIR here varies between 0.257 and 0.265, calculated using the range of average recharge 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273
temperatures in the studied fields, from 12˚C to 22˚C (Wen et al., 2018; Pinti et al., 2019a; Pinti et al., 2019b), and solubility data from Smith and Kennedy (1993). For wells AZ-83 and AZ-89, the 4He/36Ar ratio was used as an indicator of the air component, because neon was not measured (Wen et al., 2018). Using either the 4He/20Ne of the air or that of the ASW changes the Rc/Ra value obtained by less than 0.1, because the air component is negligible in our samples (less than 0.1%). Only sample AZ-66D showed air contamination (R/Ra = 1.06; not reported in Table 1), and as it was not possible to either correct the ratio or resample the well, this sample was excluded from further analyses but Cl and Br isotopes could be measured correctly in the fluid phase. Well AZ-1A was not sampled for gases, and thus only Cl and Br data from the fluid phase are reported (Table 1).
In Figs. 2a and 2b, the ranges of 37Cl and 81Br values measured in the geothermal brines extracted from production wells of the three sampled Mexican geothermal fields are reported and are compared with data from the literature. For 37Cl, data from this study were compared with those from mantle rocks and fluids (i.e., MORBs, OIBs, MOR vents) and hydrothermal and geothermal manifestations (geothermal brines of Iceland and New Zealand, hot springs, and fumaroles) (see Fig. 2a caption for references). For 81Br, none of these environments has previously been sampled, so data from this study were compared with 81Br values from
sedimentary and Precambrian shield (Canada and Fennoscandia) formation waters (Frape et al., 2007; Shouakar-Stash et al., 2007; Shouakar-Stash, 2008; Stotler et al., 2010) and from Permo-Triassic gas fields (Bagheri et al., 2014) (Fig. 2b).
The 37Cl of geothermal brines from Mexican geothermal fields was found to vary from -0.36 to +0.78‰ (Fig. 2a and Table 1), within the range measured in brines from Icelandic geothermal systems (Stefánsson et al., 2017) and in brines from Taupo, New Zealand (Bernal et 274 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296
al., 2014). Hot spring water 37Cl variability is similar to that of deep geothermal fluids, while fumaroles have extremely low or high 37Cl values, ranging from -4 to +20‰ (Fig. 2a), which has been related to distillation processes and isotopic exchange between aqueous Cl2 and gaseous HCl (Sharp et al., 2010a). The isotopic variability of Br (Fig. 2b) in sampled geothermal brines (this study) is similar to that of Cl. The measured 81Br values range from -0.58 to +0.9‰, which is within the same range as sedimentary formation brines, while brines circulating in Precambrian crystalline rocks show higher 81Br values, of up to +1.9‰ (Fig. 2b).
When Br and Cl isotopic ratios are plotted together, geothermal fluids occupy a different isotopic space than sedimentary brines (Fig. 3a). Sedimentary brines show greater isotopic variability for Br than for Cl. A rough relationship between the two halogen isotopic
compositions can be defined as 37Cl 81Br x 0.24. Shouakar-Stash (2008) suggested that the observed isotopic variability reflects local sedimentary fluid source variability. Geothermal fluids show roughly the same isotopic variability for Cl and Br (Figs. 3a, b). Another characteristic of geothermal fluids is that, except for a few outliers, the majority of sampled fluids show isotopic Cl and Br values equal to or higher than seawater, while sedimentary fluid values are both lower and higher than seawater (Fig. 3b).
5. DISCUSSION
5.1. He-Cl-Br isotopic relationships
The most striking feature is that Br and Cl isotopic variability in the geothermal fluids is related to that of helium, namely the 3He/4He ratio (Figs. 4 and 5). Helium in geothermal fluids can derive from three sources: the mantle, which is characterized by the dominance of primordial 3He over 4He, with a depleted mantle (sourcing the MORBs) 3He/4He ratio (R) that is 8±1 times 297 298 299 300 301 302 303 304 305 306 307 308 309 310 311 312 313 314 315 316 317 318 319
higher than that of the atmosphere (Ra= 1.384 x 10-6) (e.g., Allègre et al., 1995); the atmosphere or seawater, with a R/Ra of 1 by definition; and the crust, where the dominant isotope is 4He over 3He, being the decay product of U and Th contained in rocks and with typical 3He/4He ratios of 0.02 to 0.5 (Lowenstern et al., 2014). In geothermal areas, the primordial 3He isotope provides an unambiguous record of the presence of mantle volatiles (e.g., Craig et al., 1978; Mazor and Bosch, 1984; Kennedy et al., 1985; Lupton et al., 1989; Kennedy and Van Soest, 2007; Ruzié et al., 2012; Broadley et al., 2016; Pinti et al., 2017; 2019a; 2019b).
In Fig. 4, 37Cl is plotted against the 3He/4He ratio, expressed as Rc/Ra. Plotting 37Cl vs. the uncorrected R/Ra value would not substantially change the figure, given that the air
component is negligible (see the Results section). The majority of the datapoints, except for one (AZ-9A), are restricted to a near-triangular isotopic space, which suggests mixing between three distinct He and Cl isotopic sources. In Fig. 5, 81Br is also plotted against the Rc/Ra ratio, and also shows a near-triangular isotopic space, although the data are more scattered than for Cl. The reason for the Br isotopic data dispersion is not clear. It might be related to the analytical
difficulties in analyzing low-abundance Br compared to Cl. However, the higher variability in the bromine isotopic signature could explain the positive, but scattered trend between Cl and Br isotopic ratios (Fig. 3b).
The relationship between He and Cl-Br is expected, but has been rarely been researched or observed (Hulston and Lupton, 1996). Like heavy halogens (Pyle and Mather, 2009), helium is expected to be highly incompatible during mantle melting (Graham et al., 2016). While it may not be as “hydrophilic” as halogens, it is dissolved in crustal fluids where it is used to trace their sources and evolution (e.g., Ballentine et al., 2002; Genereux et al., 2009). Thus, it is expected that helium and halogens behave similarly during mantle degassing and incorporation into crustal 320 321 322 323 324 325 326 327 328 329 330 331 332 333 334 335 336 337 338 339 340 341 342
fluids. Finally, noble gases and halogens behave similarly, and their isotopic signatures are preserved during mantle processes, such as during the recycling of volatiles into the mantle (Sumino et al., 2010; Kendrick et al., 2011; Kobayashi et al., 2017).
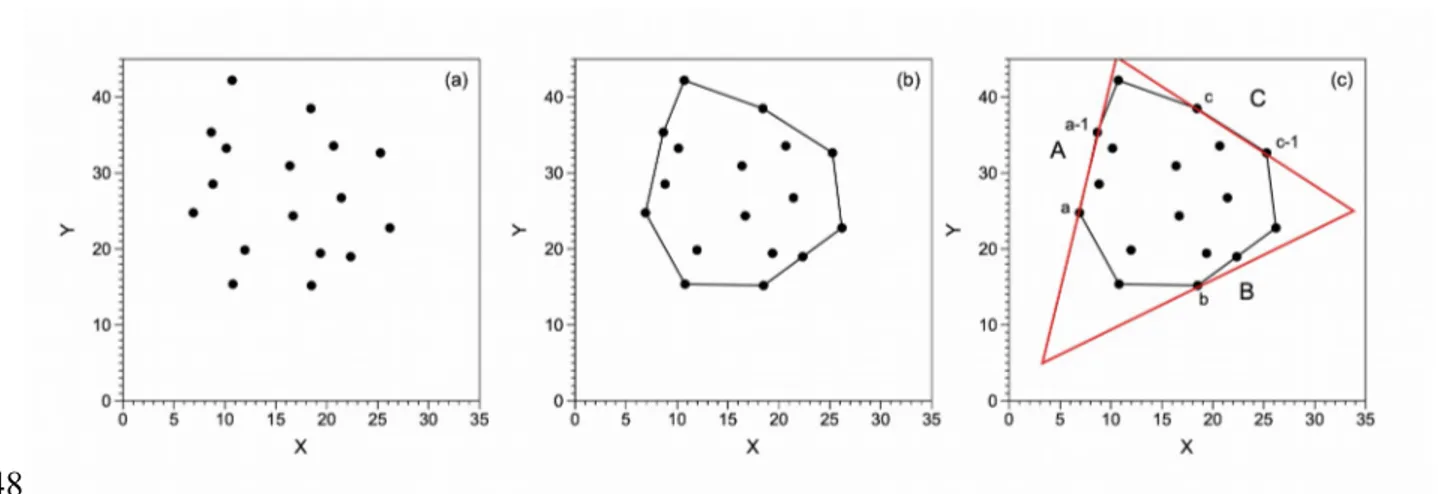
The relationship between the three isotopic systems appears to represent mixing between three distinct volatile sources (Figs. 4 and 5). To find the isotopic values of the three endmembers and the equivalent mixing triangle, we applied the algorithm of Klee and Laskowski (1985). This algorithm finds the triangle of smallest area enclosing a convex polygon – which in turn encloses scattered datapoints – in the Euclidean plane, E2. Implementation of the algorithm is presented in Pârvu and Gilbert (2016), and was compiled using Python v. 3.6 for Windows 10. Details of the mathematical treatment are reported in Appendix 1.
Figure 4 shows the mixing triangle obtained for the 37Cl – Rc/Ra plot, excluding data from AZ-9A. Three endmembers are identified. The first is associated with a depleted mantle source, with a Rc/Ra of 7.76. This value is practically indistinguishable from the value of 8±1 measured in the depleted mantle sourcing the MORBs (e.g., Allègre et al., 1995; Graham, 2002). The 37Cl value of this depleted mantle source is +0.88‰. The second endmember (labelled “Subduction”) has a Rc/Ra of 6.45 and a 37Cl of -0.43‰. The helium isotopic ratio is compatible with those observed in volcanic arc magmatism, where an average R/Ra value of 5.4±1.9 Ra has been proposed (Hilton et al., 2002). In arc volcanism, Rc/Ra values lower than those measured at the oceanic ridges, of 8±1, have often been interpreted as the (radiogenic) helium contribution from the slab into the mantle wedge (Hilton et al., 2002). The 37Cl value of -0.43‰ is consistent with that measured in porewater from the dehydration of serpentinite from the subducting slab (37Cl = -0.7±0.4‰; Bonifacie et al., 2008a). The third endmember has a Rc/Ra of 1.68 and a 37Cl of +0.11‰. The Rc/Ra ratio indicates the addition of large amount of radiogenic (crustal) 343 344 345 346 347 348 349 350 351 352 353 354 355 356 357 358 359 360 361 362 363 364 365
helium, while the 37Cl is close to that of seawater. Both He and Cl isotopic signatures suggest local crustal sources, such as poral seawater or brines. If sample AZ-9A is added, the mantle endmember would shift to a Rc/Ra of 9.90, higher than that normally expected for the depleted mantle sourcing the MORBs, and 37Cl would be +0.90‰.
Bromine shows a similar ternary mixing triangle as chlorine (Fig. 5). The depleted mantle endmember has a Rc/Ra of 8.26, again encompassing values measured in MORB lavas, and a 81Br value of +0.75‰. The endmember possibly representing the mantle wedge has a Rc/Ra of 7.17 and a 81Br value of -1.03‰. Finally, the endmember representing local crustal sources has a Rc/Ra of 1.89 and a 81Br value of ±0.26‰ (Fig. 5).
The mathematical method used to constrain the endmember isotopic compositions has some limitations. Indeed, in an isotope-isotope plot (Figs. 4 and 5), a straight line corresponds to a curvature parameter, “r”, = 1 (Langmuir et al., 1978). This means that the He/Cl ratio of the crustal source would be equal to the He/Cl ratio of the mantle sources (Figs. 4 and 5).
Interestingly, Hulston and Lupton (1996) found a similar relationship in Taupo geothermal fluids by comparing B/Cl and 3He/4He ratios. They found that the fluids were a mixture between a boron-rich crustal source and a 3He-rich mantle source, with a constant He/Cl ratio between the two sources. However, it is expected that the mixing triangles obtained in Figs. 4 and 5 are contoured by hyperbolas with “r” 1. Indeed, the mantle reservoir and Earth’s surface reservoirs should have evolved differently over the eons, in terms of their He/Cl ratios.
Why Los Azufres data trends more toward the depleted mantle endmember, while Las Tres Vírgenes data trends toward the subduction end-member, and Cerro Prieto encompasses the full range of He, Cl, and Br values (Figs. 4 and 5) remains to be resolved. The presence of nearly-pure MOR-type helium in Los Azufres can be explained by the particular geometry of the slab 366 367 368 369 370 371 372 373 374 375 376 377 378 379 380 381 382 383 384 385 386 387 388
under central Mexico. Under the TMVB (Fig. 1), the Cocos plate plunges almost vertically into the mantle (Pérez-Campos et al., 2008), rendering the direct ascent of lithospheric mantle
material underplating the region of Los Azufres possible, as suggested by the nearly-pure mantle carbon (Richard et al., 2019) and mantle 87Sr/86Sr (Wen et al., 2018; Pinti et al., 2020) of Los Azufres fluids. The high variability in helium isotopes of the two fields located in Baja California – Cerro Prieto and Las Tres Vírgenes – is more difficult to explain. Melts are expected to derive from the spreading center of the Gulf of Baja California (Fig. 1), which continues into the Cerro Prieto and Salton Sea Trough. Based on the C isotopic systematics of Las Tres Vírgenes (13C of -11to -12‰ vs PDB), Richard et al. (2019) suggested that organic-C sediments from the ancient subducting Farallon Plate have contaminated the local depleted mantle with 12C-rich and possibly 4He-rich fluids (Pinti et al., 2019b). It is worth noting that in central Baja California, a fragment of the Farallon Plate is possibly stacked into the local mantle and other fragments are possibly present in the Cerro Prieto-Salton Sea Trough area (Barak et al. 2015), thus possibly
contaminating the local melts (Batista Cruz et al., 2019).
Finally, it is important to test whether the triangular relationship observed for He, Cl, and Br represents mixing between different volatile sources or is instead created by some isotopic fractionation process in the reservoir. At first sight, because helium is an inert noble gas, high isotopic variation should be related to the isotopic composition of the volatile source, not physical processes in the reservoir, such as boiling. Indeed, any physical process of fractionation cannot account for the direct relationship between He and Br or He and Cl isotopes, because
fractionation will favor either the heavier or the lighter isotope masses. This is illustrated in Fig. 6. Here, the supposed mixing triangle between Rc/Ra (=3He/4He) and the 37Cl (=37Cl/35Cl) is reported together with an initial hypothetical datapoint, the values of which have been fixed 389 390 391 392 393 394 395 396 397 398 399 400 401 402 403 404 405 406 407 408 409 410 411
arbitrarily to Rc/Ra = 5 and 37Cl = 0‰. Arrows indicate the evolution of the hypothetical datapoint isotopic composition if some physical fractionation process were to take place in the reservoir. Reinjection of used brines would add atmospheric helium to the reservoir and lower the Rc/Ra value, but with little or no 37Cl fractionation (see section 3.). Measured 37Cl values in the reinjection fluids of Los Azufres have indeed shown values encompassing those of the production fluids. Any process involving aqueous diffusion in the reservoir will favor either the light
isotopes (in the degassed phase) or the heavier isotopes (in the residual phase). Slopes in Fig. 6 have been calculated assuming a fractionation factor of He in fluids equal to the square root of the helium masses (Marty, 1984) (D3He/D4He =0.8660) and a Cl fractionation factor for aqueous diffusion at high temperatures calculated by Eggenkamp and Coleman (2009)
(D35Cl/D37Cl=1.00192). Adiabatic boiling is not expected to fractionate the helium isotopic ratio, and modeling by Stefánsson and Barnes (2016) for Icelandic geothermal fluids has shown that 37Cl should be fractionated by less than 0.3‰. Finally, Stefánsson et al. (2017) suggested that additional 37Cl-rich chlorine sourced from HCl (gas) via magma degassing (Sharp et al., 2010a) could be introduced into geothermal systems, increasing the 37Cl to highly positive values, as observed in the Krafla geothermal field of Iceland. Again, adding 37Cl-rich HCl does not affect the helium isotopic ratio in any way (Fig. 6). In conclusion, the concomitant increase in the 3He/4He and 37Cl/35Cl ratios can only be explained by the addition of a source containing mantle helium and high 37Cl values (Fig. 6).
5.2. Br/Cl and He relationship 412 413 414 415 416 417 418 419 420 421 422 423 424 425 426 427 428 429 430 431 432
Figures 4 and 5 suggest that halogens present in the geothermal fluids of the Mexican fields are a mixture between crustal and mantle sources. The presence of these sources should also be confirmed by the elemental abundances or ratios between the two halogen species.
Pinti et al. (2019a) have shown a clear inverse relationship between the molar Br/Cl ratio and the 3He/4He ratio in Cerro Prieto fluids, discerning two distinct sources of halogens in these fluids, namely the mantle and modified seawater. If Los Azufres and Las Tres Vírgenes data are added, the inverse relationship of Pinti et al. (2019a) is again found (Fig. 7). The data can be explained by the addition of a fluid containing mantle helium, with Rc/Ra values of 6-8 and Br/Cl molar ratios lower than 0.0005, which are within the typical ranges observed in volcanoes
(Böhlke and Irwin, 1992) and hydrothermal systems (Banks et al., 2000). The second fluid in the mixture contains more crustal radiogenic 4He (Rc/Ra = 3) and a Br/Cl molar ratio of
approximately the value of seawater (0.00153) or lower (0.0011), indicating modified seawater by halite dissolution (Martini et al., 1998). It is worth noting that the He/Cl ratio between crust and mantle endmembers is different, as is expected for two reservoirs that separated earlier in the history of the Earth and evolved differently (Fig. 7).
5.3. Isotopically high Cl and Br in the depleted mantle: implications for halogen origin and cycling
This study suggests that the Cl and Br isotopic compositions of the depleted mantle are ca. 1‰ higher than those of seawater (Figs. 4 and 5), a different result from that obtained by
measuring Cl isotopes in MORBs, which yielded values from -3 to 0‰ (Sharp et al., 2007; Bonifacie et al., 2008b; Layne et al., 2009). The extremely low abundances of Cl and Br in mantle rocks is certainly an important factor, favoring contamination from several ambient 433 434 435 436 437 438 439 440 441 442 443 444 445 446 447 448 449 450 451 452 453 454 455
sources, such as seawater (Bonifacie et al., 2007; 2008b), but this cannot explain the discrepancies between our results and those of, e.g., Bonifacie et al. (2008b).
One possible explanation is that the local mantle beneath Mexico is fed by 1) isotopically light Cl and Br from serpentinite dehydration or from sediment pore water (endmember labelled “Subduction” in Figs. 4 and 5); and 2) a reservoir of isotopically heavier halogens from the metasedimentary layer on top of the subducting slab, and which would be the residual of the isotopically-depleted halogen source found in serpentinite and sediment pore water (John et al., 2010). This hypothetical reservoir has been proposed by John et al. (2010) to explain the heavier Cl isotopic signatures in primitive magmas. However, the presence of this reservoir is
inconsistent with pure MORB-like 3He/4He ratios of 7.7-8.2, determined for the “mantle”-labelled endmember of Figs. 4 and 5. The involvement of metasediments should imply the addition of radiogenic 4He, together with heavier 37Cl, which is not observed (Fig. 4).
It could be speculated that both positive (this study) and negative (e.g., Bonifacie et al., 2008b) 37Cl values are true, which would imply that the depleted mantle is heterogeneous in terms of the halogen isotopic composition. The idea of a well-stirred convective depleted mantle, where recycled volatiles are homogenously incorporated, is based on the broad homogeneity of the MORB He isotopic composition (R/Ra = 8±1; e.g., Allègre et al., 1995). Recent work by Parai and Mukhopadhyay (2015) have identified the persistence of large Ar and Xe isotopic variations in MORBs generated by heterogeneous regassing and inefficient homogenization of recycled material in the MORB source (Mukhopadhyay and Parai, 2019). It could be speculated that heavier halogens reflect this volatile isotopic heterogeneity of the mantle similarly to heavy noble gases. 456 457 458 459 460 461 462 463 464 465 466 467 468 469 470 471 472 473 474 475 476 477
Our findings presume that initial 37Cl (and by analogy 81Br) values in the mantle were positive (Fig. 8). John et al. (2010) interpreted the high 37Cl measured in OIBs from the Society Islands, of +2.9‰, as a resulting from crustal contamination by isotopically heavier halogens from subduction slab metasediments. An alternative explanation might be that the lower mantle feeding the Society Islands hotspot has (partially) preserved a high-37Cl signature of the primitive mantle (Fig. 8). Initial heavier Cl and Br isotopic compositions of the Earth are
inconsistent with chondritic precursors showing 37Cl = 0±0.7‰ (Sharp et al., 2010a), and imply that isotopic fractionation occurred during mantle degassing and the release of halogens into surface reservoirs. The Moon shows very high 37Cl values, up to +18‰ in mare basalts (e.g., Sharp et al., 2010b) and up to +40‰ in KREEK basalts (Barnes et al., 2016), which has been interpreted as resulting from the degassing of anhydrous magmas (Sharp et al., 2010b). This hypothesis has recently been challenged by Stephant et al. (2019), who concluded that the high 37Cl values in lunar basalts reflect the signature of their source region. Evaporative fractionation of halogen stable isotopes (Day and Moynier, 2014) in the Lunar Magma Ocean during the Moon-forming impact could be the cause of the primitive halogen signature in the Moon basalts (Stephant et al., 2019). Earth has retained much of its volatiles compared to the Moon, perhaps due to shielding effects from a proto-atmosphere, a stronger gravity field than on the Moon, differences in the mantle oxidation state, and/or the replenishment of volatiles to Earth after the formation of the Moon (Day and Moynier, 2014; Marty 2012). However, a smaller loss of volatiles on Earth during the Moon giant impact could have been sufficient to slightly shift the chondritic chlorine isotopic composition (37Cl 0‰) to slightly positive values in the Earth mantle ocean (37Cl +3‰), leaving a lighter degassed halogen reservoir at the surface, with 37Cl (and 81Br) close to 0‰. 478 479 480 481 482 483 484 485 486 487 488 489 490 491 492 493 494 495 496 497 498 499 500
The present-day depleted mantle Cl and Br isotopic compositions of ca. 1‰ heavier than seawater, such as that feeding the Mexican geothermal fields, would reflect a progressive (local?) decrease in 37Cl (and in 81Br) from heavier initial values of +3‰ by the continuous recycling, through subduction, of halogens with lighter isotopic signatures over the last 2-3 billion years (i.e., since global-scale plate tectonics began) (Fig. 8). The 37Cl value for the mantle wedge at subduction zones, as determined and presented in Fig. 4, is either compatible with halogen assimilation and recycling from seafloor serpentinite (-0.7±0.4‰; Bonifacie et al., 2008a) or sediment pore water (-7.8‰; Ramson et al., 1995), which have been suggested to be vehicles of halogens into the subduction zone, incorporated into serpentinite through hydration (Bonifacie et al., 2008b; John et al., 2010; Sumino et al., 2010; Kendrick et al., 2011; Kobayashi et al., 2017). Halogens released in the mantle at the final stage of serpentinite dehydration preserve their isotopic signature due to highly channelized flow (Kobayashi et al., 2017), and possibly decrease the pristine mantle heavier halogen isotopic composition to the present-day values found in the depleted mantle (Fig. 8).
6. CONCLUSIONS
This study presents the first large dataset of bromine isotopes (81Br) measured in
geothermal fluids. Together with acquired 37Cl and noble gas isotope 3He/4He ratios, these data suggest that the mantle feeding these fields has a halogen isotopic composition ca. +1‰ higher than seawater, challenging previous perspectives on halogen addition to Earth and halogen cycling into the deep mantle. It is unclear at this stage whether the obtained results reflect a local mantle beneath Mexico or inefficient homogenization of recycled material in the MORB source, as suggested elsewhere from the compositions of heavier noble gases, Ar and Xe, in mantle 501 502 503 504 505 506 507 508 509 510 511 512 513 514 515 516 517 518 519 520 521 522 523
rocks. Heterogeneous regassing of isotopically light halogens into an initial isotopically heavier mantle may be responsible for either positive or negative Cl and Br isotopic values, as observed in this and other studies on mantle rocks. Certainly, these results bring to light the necessity of obtaining fresh data from geothermal fluids from different tectonic settings, such as
subcontinental mantle, intraplate, and spreading settings, so as to better constrain whole-Earth halogen isotopic systematics.
ACKNOWLEDGMENTS
This manuscript is dedicated to the memory of Prof. Victor-Hugo Garduño-Monroy, without whom it would not be possible. D.L.P. wishes to thank M. Coleman for early comments on the manuscript and Long Li access to his literature database. Thanks to H. Bureau for
encouragement, comments, and suggestions, and for, together with H. Balcone-Boissard, organizing the international meeting on halogens Cycl’Hal at Sorbonne Université, which benefited D.L.P. through fruitful discussions with colleagues, including R. Burgess and M. Bonifacie. We wish to thank the handling editor and three anonymous reviewers for the fruitful and thoughtful comments that have greatly improved this manuscript. PhD L. Daver is thanked for illustrating our hypotheses in Figure 8. This project was funded by CeMieGEO P-20 (Call FSE-SENER-CONACyT: 2013-01, project N°207032) to A.L.-H; NSF Instrumentation & Facilities award EAR-1049822 to M.C.C.; and NSERC Discovery Grant (RGPIN-2015-05378) to D.L.P. 524 525 526 527 528 529 530 531 532 533 534 535 536 537 538 539 540 541 542 543 544 545
References
Allègre C.J., Moreira M., Staudacher T. (1995). 4He/3He dispersion and mantle convection. Geophys. Res. Lett. 22, 2325–2328.
Arango-Galván C., Prol-Ledesma R.M. and Torres-Vera M.A. (2015). Geothermal prospects in the Baja California Peninsula. Geothermics 55, 39-57.
Arnórsson, S, Stefánsson, A., Bjarnason, J.Ö., 2007. Fluid-fluid interactions in geothermal systems. Rev. Mineral. Geochem. 65, 259-312.
Bagheri R., Nadri A., Raeisi E., Eggenkamp H.G.M., Kazemi G.A. and Montaseri A. (2014). Hydrochemical and isotopic (δ18O, δ2H, 87Sr/86Sr, δ37Cl and δ81Br) evidence for the origin of saline formation water in a gas reservoir. Chem. Geol. 384, 62-75.
Ballentine C.J., Burgess R. and Marty B. (2002). Tracing fluid origin, transport and interaction in the crust. Rev. Mineral. Geochem. 47, 539-614.
Banks D.A., Gleeson S.A. and Green R. (2000). Determination of the origin of salinity in granite related fluids: evidence from chlorine isotopes in fluid inclusions. J. Geochem. Explor.
69-70, 309-312.
Barak S., Klemperer S.L. and Lawrence J.F. (2015). San Andreas Fault dip, Peninsular Ranges mafic lower crust and partial melt in the Salton Trough, Southern California, from ambient-noise tomography. Geochem. Geophys. Geosyst. 16, 3946-3972.
Barnes J.D., Sharp Z.D. and Fischer T.P. (2008). Chlorine isotope variations across the Izu-Bonin-Mariana arc. Geology 36, 883-886.
Barnes J.D., Sharp Z.D., Fischer T.P., Hilton D.R. and Carr M.J. (2009). Chlorine isotope variations along the Central American volcanic front and back arc. Geochem. Geophys. Geosyst. 10 Q11S17, doi:10.1029/2009GC002587. 546 547 548 549 550 551 552 553 554 555 556 557 558 559 560 561 562 563 564 565 566 567 568
Barnes J.D., Prather T.J., Cisneros M., Befus K., Gardner J.E. and Larson T.E. (2014). Stable chlorine isotope behavior during volcanic degassing of H2O and CO2 at Mono Craters, CA. Bull. Volcanol. 76, 805.
Barnes J.J., Tartèse R., Anand M., McCubbin F.M., Neal C.R. and Franchi I.A., 2016. Early degassing of lunar urKREEP by crust-breaching impact(s). Earth Planet. Sci. Lett. 447, 84-94.
Barragán R.M., Iglesias E.R., Torres R.J., Arellano V.M., Reyes-Picasso N., Ramírez, M., Tapia R. and Hernández P. (2009). Mixing and dilution processes at the Las Tres Vírgenes (México) geothermal reservoir indicated by 1997-2007 geochemical data. GRC Trans. 33, 43-48.
Batista Cruz R.Y., Rizzo A.L., Grassa F., Bernard Romero R., González Fernández A.,
Kretzschmar T.G. and Gómez-Arias E. (2019). Mantle degassing through continental crust triggered by active faults: the case of the Baja California Peninsula, Mexico. Geochem. Geophys. Geosyst. 20, 1912-1936.
Bernal N.F., Gleeson S.A., Dean A.S., Liu X.-M. and Hoskin P. (2014). The source of halogens in geothermal fluids from the Taupo Volcanic Zone, North Island, New Zealand. Geochim. Cosmochim. Acta 126, 265-283.
Birkle P., Merkel B., Portugal E. and Torres-Alvarado I.S. (2001). The origin of reservoir fluids in the geothermal field of Los Azufres, Mexico — isotopical and hydrological indications. Appl. Geochem. 16, 1595-1610.
Böhlke J.K. and Irwin J.J. (1992). Laser microprobe analyses of Cl, Br, I, and K in fluid inclusions: Implications for sources of salinity in some ancient hydrothermal fluids. Geochim. Cosmochim. Acta 56, 203-225.
569 570 571 572 573 574 575 576 577 578 579 580 581 582 583 584 585 586 587 588 589 590 591
Bonifacie M., Charlou J.L., Jendrzejewski N., Agrinier P. and Donval J.P. (2005). Chlorine isotopic compositions of high temperature hydrothermal vent fluids over ridge axes. Chem. Geol. 221, 279-288.
Bonifacie M., Monnin C., Jendrzejewski N., Agrinier P. and Javoy M. (2007). Chlorine stable isotopic composition of basement fluids of the eastern flank of the Juan de Fuca Ridge (ODP Leg 168). Earth Planet. Sci. Lett. 260, 10-22.
Bonifacie M., Busigny V., Mével C., Philippot P., Agrinier P., Jendrzejewski N., Scambelluri M. and Javoy M. (2008a). Chlorine isotopic composition in seafloor serpentinites and high-pressure metaperidotites. Insights into oceanic serpentinization and subduction processes. Geochim. Cosmochim. Acta 72, 126-139.
Bonifacie M., Jendrzejewski N., Agrinier P., Humler E., Coleman M. and Javoy M. (2008b). The chlorine isotope composition of Earth’s mantle. Science 319, 1518-1520.
Broadley M.W., Ballentine C.J., Chavrit D., Dallai L. and Burgess R. (2016). Sedimentary halogens and noble gases within Western Antarctic xenoliths: Implications of extensive volatile recycling to the sub continental lithospheric mantle. Geochim. Cosmochim. Acta
176, 139-156.
Burgess R., Layzelle E., Turner G. and Harris J.W. (2002). Constraints on the age and halogen composition of mantle fluids in Siberian coated diamonds. Earth Planet. Sci. Lett. 197, 193-203.
Clay P.L., Burgess R., Busemann H., Ruzié-Hamilton L., Joachim B., Day J.M.D., Ballentine C.J (2017). Halogens in chondritic meteorites and terrestrial accretion. Nature 551, 614-618. Craig H., Lupton J.E., Welhan J.A. and Poreda R. (1978). Helium isotope ratios in Yellowstone
and Lassen Park volcanic gases. Geophys. Res. Lett. 5, 897-900. 592 593 594 595 596 597 598 599 600 601 602 603 604 605 606 607 608 609 610 611 612 613 614
Cullen J.T., Barnes J.D., Hurwitz S. and Leeman W.P. (2015). Tracing chlorine sources of thermal and mineral springs along and across the Cascade Range using halogen
concentrations and chlorine isotope compositions. Earth Planet. Sci. Lett. 426, 225-234. Day J.M.D. and Moynier F. (2014). Evaporative fractionation of volatile stable isotopes and their
bearing on the origin of the Moon. Phil. Trans. Royal Soc. A 372, 20130259.
Eggenkamp H.G.M. (2014). The Geochemistry of Stable Chlorine and Bromine Isotopes. Springer, Heidelberg New York Dordrecht London.
Eggenkamp H.G.M. and Coleman M.L. (2000). Rediscovery of classical methods and their application to the measurement of stable bromine isotopes in natural samples. Chem. Geol.
167, 393-402.
Eggenkamp H.G.M. and Coleman M.L. (2009). The effect of aqueous diffusion on the fractionation of chlorine and bromine stable isotopes. Geochim. Cosmochim. Acta 73, 3539-3548.
Elders W.A., Bird D.K., Williams A.E. and Schiffman P. (1984). Hydrothermal flow regime and magmatic heat source of the Cerro Prieto geothermal system, Baja California, Mexico. Geothermics 13, 27-47.
Ferrari L., Orozco-Esquivel T., Manea V. andManea M. (2012). The dynamic history of the Trans-Mexican Volcanic Belt and the Mexico subduction zone. Tectonophysics 522-523, 122-149.
Frape S.K., Shouakar-Stash O., Pačes T. and Stotler R. (2007). Geochemical and isotopic characteristics of the waters from crystalline and sedimentary structures of the Bohemian Massif. In: Bullen T, editor. 12th International Symposium on Water Rock Interaction, 727-733. 615 616 617 618 619 620 621 622 623 624 625 626 627 628 629 630 631 632 633 634 635 636 637
García-Sánchez L., Macías J.L., Sosa-Ceballos G., Arce J.L., Garduño-Monroy V.H., Saucedo R., Avellán D.R., Rangel E., Layer P.W., López-Loera H., Rocha V.S., Cisneros G., Reyes-Agustín G., Jiménez A. and Benowitz J.A. (2017). Genesis and evolution of the Cerro Prieto Volcanic Complex, Baja California, Mexico. Bull. Volcanol. 79, 44-53.
Genereux D.P., Webb M. and Solomon D.K. (2009). Chemical and isotopic signature of old groundwater and magmatic solutes in a Costa Rican rain forest: Evidence from carbon, helium, and chlorine. Water Resour. Res. 45, W08413.
Giggenbach W.F. (1992). Isotopic shifts in waters from geothermal and volcanic systems along convergent plate boundaries and their origin. Earth Planet. Sci. Lett. 113, 495-510.
Godon A.R., Jendrzejewski N., Eggenkamp H.G.M., Banks D.A., Ader M., Coleman M.L. and Pineau F. (2004). A cross-calibration of chlorine isotopic measurements and suitability of seawater as the international reference material. Chem. Geol. 207, 1-12.
Graham D.W. (2002). Noble gas isotope geochemistry of mid-ocean ridge and ocean island basalts: characterization of mantle source reservoirs. Rev. Mineral. Geochem. 47, 247-317. Graham D.W., Michael P.J. and Shea T. (2016). Extreme incompatibility of helium during
mantle melting: Evidence from undegassed mid-ocean ridge basalts. Earth Planet. Sci. Lett. 454, 192-202.
Gutiérrez-Negrín L.C.A. (2015). Cerro Prieto, Mexico - A convective extensional geothermal play. GRC Trans. 39, 711-716.
Hedenquist J.W. and Lowenstern J.B. (1994). The role of magmas in the formation of hydrothermal ore deposits. Nature 370, 519-527.
Hilton D.R., Fischer T.P. and Marty B. (2002). Noble gases and volatile recycling at subduction zones. Rev. Mineral. Geochem. 47, 319-370.
638 639 640 641 642 643 644 645 646 647 648 649 650 651 652 653 654 655 656 657 658 659 660
Hulston J.R. and Lupton J.E. (1996). Helium isotope studies of geothermal fields in the Taupo Volcanic Zone, New Zealand. J. Volcanol. Geother. Res. 74, 297-321.
Jambon A., Déruelle B., Dreibus G. and Pineau F. (1995). Chlorine and bromine abundance in MORB: the contrasting behaviour of the Mid-Atlantic Ridge and East Pacific Rise and implications for chlorine geodynamic cycle. Chem. Geol. 126, 101-117.
John T., Layne G.D., Haase K.M. and Barnes J.D. (2010). Chlorine isotope evidence for crustal recycling into the Earth's mantle. Earth Planet. Sci. Lett. 298, 175-182.
Kendrick M.A., Scambelluri M., Honda M. and Phillips D. (2011). High abundances of noble gas and chlorine delivered to the mantle by serpentinite subduction. Nature Geosci. 4, 807-812. Kennedy B.M., Lynch M.A., Reynolds J.H. and Smith S.P. (1985). Intensive sampling of noble
gases in fluids at Yellowstone: I. Early overview of the data; regional patterns. Geochim. Cosmochim. Acta 49, 1251-1261.
Kennedy B.M. and van Soest M.C. (2007). Flow of mantle fluids through the ductile lower crust: Helium isotope trends. Science 318, 1433-1436.
Kobayashi M., Sumino H., Nagao K., Ishimaru S., Arai S., Yoshikawa M., Kawamoto T., Kumagai Y, Burgess R. and Ballentine C.J. (2017). Slab-derived halogens and noble gases illuminate closed system processes controlling volatile element transport into the mantle wedge. Earth Planet. Sci. Lett. 457, 106-116.
Langmuir D., Vocke R.D. Hanson G.N. and Hart S. (1978). A general mixing equation with applications to Iceland basalts. Earth Planet. Sci. Lett. 37, 380-392.
Layne G.D., Kent A.J.R. and Bach W. (2009). δ37Cl systematics of a back-arc spreading system: The Lau Basin. Geology 37, 427-430.
Klee V. and Laskowski M.C. (1985). Finding the smallest triangles containing a given convex 661 662 663 664 665 666 667 668 669 670 671 672 673 674 675 676 677 678 679 680 681 682 683
Li L., Bonifacie M., Aubaud C., Crispi O., Dessert C. and Agrinier P. (2015). Chlorine isotopes of thermal springs in arc volcanoes for tracing shallow magmatic activity. Earth Planet. Sci. Lett. 413, 101-110.
Lira Herrera H. (2005). Actualización del modelo geológico conceptual del yacimiento geotérmico de Cerro Prieto B.C. Geotermia 18, 37-46.
Louvat P., Bonifacie M., Giunta T., Michel A., Coleman M. (2016). Determination of Bromine stable isotope ratios from saline solutions by « wet plasma » MC-ICPMS including a comparison between high- and low-resolution modes, and three introduction systems. Anal. Chem. 88, 3891-3898.
Lowenstern J.B., Evans W.C., Bergfeld D. and Hunt A.G. (2014). Prodigious degassing of a billion years of accumulated radiogenic helium at Yellowstone. Nature 506, 355-358. Lupton J.E., Baker E.T., Massoth G.J. (1989). Variable 3He/heat ratios in submarine
hydrothermal systems: evidence from two plumes over the Juan de Fuca ridge. Nature 337, 161-164.
Martini A.M., Walter L.M., Budai J.M., Ku T.C.W., Kaiser C.J. and Schoell M. (1998). Genetic and temporal relations between formation waters and biogenic methane: Upper Devonian Antrim Shale, Michigan Basin, USA. Geochim. Cosmochim. Acta 62, 1699-1720.
Marty B. (2012). The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth Planet. Sci. Lett. 313–314, 56-66.
Mazor E. and Truesdell A.H. (1984). Dynamics of a geothermal field traced by noble gases: Cerro Prieto, Mexico. Geothermics 13, 91–102.
McDonough W.F. (2003(. Compositional Model for the Earth's Core, in: Holland, H.D., Turekian, K.K. (Eds.), Treatise on Geochemistry. Pergamon, Oxford, pp. 547-568. 685 686 687 688 689 690 691 692 693 694 695 696 697 698 699 700 701 702 703 704 705 706 707
Moeck I. and Beardsmore G. (2014). A new ‘geothermal play type’ catalog: Streamlining exploration decision making. Proc. Thirty-Ninth Workshop Geother. Res. Engin. Stanford Un., Stanford, California, February 24-26, 2014, SGP-TR-202, p.8.
Mukhopadhyay S. and Parai R. (2019). Noble gases: a record of Earth's evolution and mantle dynamics. Annu. Rev. Earth Planet. Sci. 47, 389-419.
Ozima M., Podosek F.A. (1983). Noble Gas Geochemistry. Cambridge University Press, Cambridge.
Parai R. and Mukhopadhyay S. (2015). The evolution of MORB and plume mantle volatile budgets: constraints from fission Xe isotopes in Southwest Indian Ridge basalts. Geochem. Geophys. Geosyst. 16, 719–35.
Pârvu O. and Gilbert D. (2016). Implementation of linear minimum area enclosing triangle algorithm. Comput. Appl. Math. 35, 423-438.
Pérez H., Macías J.L., Garduño V.H., Arce J.L., García F., Castro R., Layer P., Saucedo R., Martínez C., Jiménez A., Valdes G., Meriggi L. and Hernández R. (2010). Estudio vulcanológico y structural de la secuencia estratigráfica Mil Cumbres y del campo geotérmico de Los Azufres, Mich. Geotermia 23, 51-63.
Pérez-Campos X., Kim Y., Husker A., Davis Paul M., Clayton R.W., Iglesias A., Pacheco Javier F., Singh Shri K., Manea Vlad C. and Gurnis M. (2008). Horizontal subduction and
truncation of the Cocos Plate beneath central Mexico. Geophys. Res. Lett. 35, L18303. Pinti D.L., Castro M., Shouakar-Stash O., Tremblay A., Garduño V., Hall C., Hélie J.-F. and
Ghaleb B. (2013). Evolution of the geothermal fluids at Los Azufres, Mexico, as traced by noble gas isotopes, δ18O, δD, δ13C and 87Sr/86Sr. J. Volcanol. Geother. Res. 249, 1-11.
708 709 710 711 712 713 714 715 716 717 718 719 720 721 722 723 724 725 726 727 728 729
Pinti D.L., Castro M.C., Lopez-Hernández A., Han G., Shouakar-Stash O., Hall C.M. and Ramírez-Montes M. (2017). Fluid circulation and reservoir conditions of the Los Humeros Geothermal Field (LHGF), Mexico, as revealed by a noble gas survey. J. Volcanol. Geother. Res. 333, 104-115.
Pinti D.L., Castro M.C., López-Hernández A., Hernández Hernández M.A., Richard L., Hall C.M., Shouakar-Stash O., Flores-Armenta M. and Rodríguez-Rodríguez M.H. (2019a). Cerro Prieto Geothermal Field (Baja California, Mexico) – A fossil system? Insights from a
noble gas study. J. Volcanol. Geother. Res. 371, 32-45.
Pinti D.L., Castro M.C., López-Hernández A., Hernández Hernández M.A., Shouakar-Stash O., Richard L., Nuñez-Hernández S., Hall C.M. and Ramírez-Montes M. (2019b). Signature of ongoing brine reinjection on noble gas isotopes and fluid chemistry at Las Tres Vírgenes geothermal field, Mexico. J. Volcanol. Geother. Res. 377, 33–42.
Pinti D.L., Castro M.C., López-Hernández A., Shouakar-Stash O., Hall C.M., Richard L., Hernández Hernández M.A., Nuñez-Hernández S., Ramirez-Montes M. and Sandoval-Medina F. (2020). Isotopic Footprint of Mexican Geothermal Fluids: Constraining the Tectonic Settings. Proc. World Geother. Congr. 2020, Reykjavik, Iceland, April 26 – May 2, 2020, 1-10.
Pyle D.M. and Mather T.A. (2009). Halogens in igneous processes and their fluxes to the atmosphere and oceans from volcanic activity: A review. Chem. Geol. 263, 110-121. Ransom B., Spivack A.J. and Kastner M. (1995). Stable Cl isotopes in subduction-zone pore
waters: Implications for fluid-rock reactions and the cycling of chlorine. Geology 23, 715-718. 730 731 732 733 734 735 736 737 738 739 740 741 742 743 744 745 746 747 748 749 750 751
Rizzo A.L., Caracausi A., Liotta M., Paonita A., Barnes J.D., Corsaro R.A. and Martelli M. (2013). Chlorine isotope composition of volcanic gases and rocks at Mount Etna (Italy) and inferences on the local mantle source. Earth Planet. Sci. Lett. 371-372, 134-142.
Ruzié L., Moreira M. and Crispi O. (2012). Noble gas isotopes in hydrothermal volcanic fluids of La Soufrière volcano, Guadeloupe, Lesser Antilles arc. Chem. Geol. 304-305, 158-165. Shields W.R., Garner E.L. and Dibeler V.H. (1963). Absolute isotopic abundance ratio and the
atomic weight of chlorine, in: Elliott, R.M. (Ed.), Advances in Mass Spectrometry. Pergamon, pp. 163-173.
Schilling J.G., Unni C.K. and Bender M.L. (1978). Origin of chlorine and bromine in the oceans. Nature 273, 631-636.
Sharp Z.D., Barnes J.D., Brearley A.J., Chaussidon M., Fischer T.P. and Kamenetsky V.S. (2007). Chlorine isotope homogeneity of the mantle, crust and carbonaceous chondrites. Nature 446, 1062-1065.
Sharp Z.D., Barnes J.D., Fischer T.P. and Halick M. (2010a). An experimental determination of chlorine isotope fractionation in acid systems and applications to volcanic fumaroles. Geochim. Cosmochim. Acta 74, 264-273.
Sharp Z.D., Shearer C.K., McKeegan K.D., Barnes J.D. and Wang Y.Q. (2010b). The chlorine isotope composition of the Moon. Science 329,1050–1053.
Shouakar-Stash O. (2008). Evaluation of Stable Chlorine and Bromine Isotopes in Sedimentary Formation Fluids. PhD thesis, University of Waterloo, Waterloo, Canada. Available open access at http://hdl.handle.net/10012/3717.
Shouakar-Stash O., Drimmie R.J. and Frape S.K. (2005a). Determination of inorganic chlorine stable isotopes by continuous flow isotope ratio mass spectrometry. Rapid Comm. Mass 752 753 754 755 756 757 758 759 760 761 762 763 764 765 766 767 768 769 770 771 772 773 774
Shouakar-Stash O., Frape S.K. and Drimmie R.J. (2005b). Determination of Bromine stable isotopes using continuous-flow isotope ratio mass spectrometry. Anal. Chem. 77, 4027-4033.
Shouakar-Stash O., Alexeev S.V., Frape S.K., Alexeeva L.P. and Drimmie R.J. (2007).
Geochemistry and stable isotopic signatures, including chlorine and bromine isotopes, of the deep groundwaters of the Siberian Platform, Russia. Appl. Geochem. 22, 589-605. Smith S.P. and Kennedy B.M. (1983). The solubility of noble gases in water and in NaCl brine.
Geochim. Cosmochim. Acta 47, 503–515.
Stefánsson A. and Barnes J.D. (2016). Chlorine isotope geochemistry of Icelandic thermal fluids: Implications for geothermal system behavior at divergent plate boundaries. Earth Planet. Sci. Lett. 449, 69-78.
Stefánsson A., Hilton D.R., Sveinbjörnsdóttir Á.E., Torssander P., Heinemeier J., Barnes J.D., Ono S., Halldórsson S.A., Fiebig J. and Arnórsson S. (2017). Isotope systematics of Icelandic thermal fluids. J. Volcanol. Geother. Res. 337, 146-164.
Stephant A., Anand M., Zhao X., Chan Q.H.S., Bonifacie M. and Franchi I.A. (2019). The chlorine isotopic composition of the Moon: Insights from melt inclusions. Earth Planet. Sci. Lett. 523, 115715.
Stotler R.L., Frape S.K. and Shouakar-Stash O. (2010). An isotopic survey of δ81Br and δ37Cl of dissolved halides in the Canadian and Fennoscandian Shields. Chem. Geol. 274, 38-55. Suárez-Vidal F., Mendoza-Borunda R., Nafarrete-Zamarripa L.M., Rámirez J. and Glowacka E.
(2008). Shape and dimensions of the Cerro Prieto pull-apart basin, Mexicali, Baja California, Mexico, based on the regional seismic record and surface structures. Intern. Geol. Rev. 50, 636-649. 776 777 778 779 780 781 782 783 784 785 786 787 788 789 790 791 792 793 794 795 796 797 798
Sumino H., Burgess R., Mizukami T., Wallis S.R., Holland G. and Ballentine C.J. (2010). Seawater-derived noble gases and halogens preserved in exhumed mantle wedge peridotite. Earth Planet. Sci. Lett. 294, 163-172.
Tello-López M.R. and Torres-Rodríguez M.A. (2015). Behavior of the production characteristics of the wells in the Las Tres Vírgenes, B.C.S., Geothermal Field, México. Proc. World Geothermal Congress 2015; Melbourne, Australia.
Torres-Rodríguez M.A., Mendoza-Covarrubias A. and Medina-Martinez M. (2005). An Update of the Los Azufres Geothermal Field, after 21 Years of Exploitation. Proc. World
Geothermal Congress 2005; 2005; Antalya, Turkey, p. 1-8.
Wen T., Pinti D.L., Castro M.C., López-Hernández A., Hall C.M., Shouakar-Stash O., Sandoval-Medina, F. (2018). A noble gas and 87Sr/86Sr study in fluids of the Los Azufres geothermal field, Mexico – Assessing impact of exploitation and constraining heat sources. Chem. Geol. 483, 426-441.
Yardley B.W.D. and Bodnar R.J., 2014. Fluids in the Continental Crust. Geochem. Persp. 3, pp. 127. 799 800 801 802 803 804 805 806 807 808 809 810 811 812 813 814