© Yanina Mircheva, 2020
Slow Inhibition and Inhibitory Recruitment in the
Hippocampal Dentate Gyrus
Thèse
Yanina Mircheva
Doctorat en neurobiologie
Philosophiæ doctor (Ph. D.)
Slow Inhibition and Inhibitory Recruitment in
the Hippocampal Dentate Gyrus
Thèse
Yanina Mircheva
Sous la direction de:
ii
Résumé
L’hippocampe joue un rôle central dans la navigation spatiale, la mémoire et l’organisation spatio-temporelle des souvenirs. Ces fonctions sont maintenues par la capacite du gyrus denté (GD) de séparation des patrons d'activité neuronales. Le GD est situé à l’entrée de la formation hippocampique où il reconnaît la présence de nouveaux motifs parmi la densité de signaux afférant arrivant par la voie entorhinale (voie perforante). Le codage parcimonieux est la marque distinctive du GD. Ce type de codage est le résultat de la faible excitabilité intrinsèque des cellules granulaires (CGs) en combinaison avec une inhibition locale prédominante. En particulier, l’inhibition de type « feedforward » ou circuit inhibiteur antérograde, est engagée par la voie perforante en même temps que les CGs. Ainsi les interneurones du circuit antérograde fournissent des signaux GABAergique aux CGs de manière presque simultanée qu’elles reçoivent les signaux glutamatergiques. Cette thèse est centrée sur l’étude des interactions entre ces signaux excitateurs de la voie entorhinale et les signaux inhibiteurs provenant des interneurones résidant dans le GD et ceci dans le contexte du codage parcimonieux et le patron de décharge en rafale caractéristique des cellules granulaires.
Nous avons adressé les relations entre les projections entorhinales et le réseau inhibitoire antérograde du GD en faisant des enregistrements électrophysiologiques des CG pendant que la voie perforante est stimulée de manière électrique ou optogénétique. Nous avons découvert un nouvel mécanisme d’inhibition qui apparait à délais dans les CGs suite à une stimulation dans les fréquences gamma. Ce mécanisme induit une hyperpolarisation de longue durée (HLD) et d’une amplitude prononce. Cette longue hyperpolarisation est particulièrement prolongée et dépasse la durée d’autres types d’inhibition transitoire lente décrits chez les CGs. L’induction de HLD crée une fenêtre temporaire de faible excitabilité suite à laquelle le patron de décharge des CGs et l’intégration d’autres signaux excitateurs sont altérés de manière transitoire. Nous avons donc conclu que l’activité inhibitrice antérograde joue un rôle central dans les processus de codage dans le GD. Cependant, alors qu’il existe une multitude d’études décrivant les interneurones qui font partie de ce circuit inhibiteur, la question de comment ces cellules sont recrutées par la voie entorhinale reste quelque peu explorée. Pour apprendre plus à ce sujet, nous avons enregistré des interneurones résidant
iii
dans la couche moléculaire du GD tout en stimulant la voie perforante de manière optogénétique. Cette méthode de stimulation nous a permis d’induire la libération de glutamate endogène des terminales entorhinales et ainsi d’observer le recrutement purement synaptique d’interneurones. De manière surprenante, les résultats de cette expérience démontrent un faible taux d’activation des interneurones, accompagné d’un tout aussi faible nombre total de potentiels d’action émis en réponse à la stimulation même à haute fréquence. Ce constat semble contre-intuitif étant donné qu’en générale on assume qu’une forte activité inhibitrice est requise pour le maintien du codage parcimonieux. Tout de même, l’analyse des patrons de décharge des interneurones qui ont été activés a fait ressortir la prééminence de trois grands types: décharge précoce, retardée ou régulière par rapport le début des pulses lumineux.
Les résultats obtenus durant cette thèse mettent la lumière sur l’important conséquences fonctionnelles des interactions synaptique et polysynaptique de nature transitoire dans les réseaux neuronaux. Nous aimerions aussi souligner l’effet prononcé de l’inhibition à court terme du type prolongée sur l’excitabilité des neurones et leurs capacités d’émettre des potentiels d’action. De plus que cet effet est encore plus prononcé dans le cas de HLD dont la durée dépasse souvent la seconde et altère l’intégration d’autres signaux arrivants simultanément. Donc on croit que les effets de HLD se traduisent au niveau du réseaux neuronal du GD comme une composante cruciale pour le codage parcimonieux. En effet, ce type de codage semble être la marque distinctive de cette région étant donné que nous avons aussi observé un faible niveau d’activation chez les interneurones. Cependant, le manque d’activité accrue du réseau inhibiteur antérograde peut être compensé par le maintien d’un gradient GABAergique constant à travers le GD via l’alternance des trois modes de décharges des interneurones. En conclusion, il semble que le codage parcimonieux dans le GD peut être préservé même en absence d’activité soutenue du réseau inhibiteur antérograde et ceci grâce à des mécanismes alternatives d’inhibition prolongée à court terme.
iv
Abstract
The hippocampus is implicated in spatial navigation, the generation and recall of memories, as well as their spatio-temporal organization. These functions are supported by the processes of pattern separation that occurs in the dentate gyrus (DG). Situated at the entry of the hippocampal formation, the DG is well placed to detect and sort novelty patterns amongst the high-density excitatory signals that arrive via the entorhinal cortex (EC). A hallmark of the DG is sparse encoding that is enabled by a combination of low intrinsic excitability of the principal cells and local inhibition. Feedforward inhibition (FFI) is recruited directly by the EC and simultaneously with the granule cells (GCs). Therefore, FFI provides fast GABA release and shapes input integration at the millisecond time scale. This thesis aimed to investigate the interplay of entorhinal excitatory signals with GCs and interneurons, from the FFI in the DG, in the framework of sparse encoding and GC’s characteristic burst firing. We addressed the long-range excitation – local inhibitory network interactions using electrophysiological recordings of GCs – while applying an electrical or optogenetic stimulation of the perforant path (PP) in the DG. We discovered and described a novel delayed-onset inhibitory post synaptic potential (IPSP) in GCs, following PP stimulation in the gamma frequency range. Most importantly, the IPSP was characterized by a large amplitude and prolonged decay, outlasting previously described slow inhibitory events in GCs. The long-lasting hyperpolarization (LLH) caused by the slow IPSPs generates a low excitability time window, alters the GCs firing pattern, and interferes with other stimuli that arrive simultaneously. FFI is therefore a key player in the computational processes that occurs in the DG. However, while many studies have been dedicated to the description of the various types of the interneurons from the FFI, the question of how these cells are synaptically recruited by the EC remains not entirely elucidated. We tackled this problem by recording from interneurons in the DG molecular layer during PP-specific optogenetic stimulation. Light-driven activation of the EC terminals enabled a purely synaptic recruitment of interneurons via endogenous glutamate release. We found that this method of stimulation recruits only a subset of interneurons. In addition, the total number of action potentials (AP) was surprisingly low even at high frequency stimulation. This result is counterintuitive, as strong and persistent inhibitory signals are assumed to restrict GC
v
activation and maintain sparseness. However, amongst the early firing interneurons, late and regular spiking patterns were clearly distinguishable. Interestingly, some interneurons expressed LLH similar to the GCs, arguing that it could be a commonly used mechanism for regulation of excitability across the hippocampal network.
In summary, we show that slow inhibition can result in a prolonged hyperpolarization that significantly alters concurrent input’s integration. We believe that these interactions contribute to important computational processes such as sparse encoding. Interestingly, sparseness seems to be the hallmark of the DG, as we observed a rather low activation of the interneuron network as well. However, the alternating firing of ML-INs could compensate the lack of persistent activity by the continuous GABA release across the DG. Taken together these results offer an insight into a mechanism of feedforward inhibition serving as a sparse neural code generator in the DG.
vi
Table of Contents
Résumé ... ii Abstract ... iv Table of Contents ... vi List of Figures ... xiList of Tables ... xii
Abbreviations ... xiii
Acknowledgements ... xvii
Foreword ... xix
General Introduction ... 1
Chapter 1. Communication in the Neuronal Circuits ... 3
1.1. Primitive communication and specialization of the neural cells ... 3
1.2. Modern brain specializations ... 5
1.3. Specialization of brain areas ... 6
1.4. Specialization of the neural networks ... 7
1.5. Study of the neural circuits ... 7
1.6. Mapping the brain circuits – combined techniques for system neuroscience studies ... 15
1.7. Hippocampus – highlight over the Dentate gyrus ... 19
1.8. The Dentate Gyrus ... 34
1.9. Communication in the DG micro-circuit... 44
1.10. Scientific context and thesis objectives ... 61
Chapter 2. Materials and Methods ... 64
vii
• Animals ... 64
• Slice preparation ... 64
• Electrophysiological recordings ... 64
2.2. Immunostainings and imaging ... 65
• Biocytin staining ... 65 • Calbindin staining ... 66 • Fluorescent images ... 66 2.3. Pharmacology ... 66 2.4. Intracranial surgeries ... 67 • Troubleshooting ... 67
• Stereotaxic injections from bregma ... 67
2.5. Optogenetic manipulation ... 71
2.6. Experimental design and Analysis ... 72
Chapter 3. Interplay of entorhinal input and local inhibitory network in the hippocampus at the origin of slow inhibition in granule cells ... 73
3.1. Résumé ... 73
3.2. Abstract ... 74
3.3. Introduction ... 74
3.4. EPSPs evoked by perforant path stimulation are followed by a long-lasting hyperpolarization (LLH) in dentate GCs. ... 76
3.5. LLH is dependent on mixed GABAA/GABAB receptors (GABAA/B-R) and metabotropic glutamate receptors (mGluR2) activation. ... 79
3.6. LLH is an age-dependent mechanism. ... 86
3.7. LLH is activity-dependent. ... 88
3.8. LLH is induced through synaptic activation of feedforward inhibition by the perforant path. ... 91
viii
3.9. LLH bursts restrain the firing time window in response to theta frequency activity of
the same input and/or upcoming signals from other inputs. ... 93
3.10. Conclusion and Discussion ... 99
3.10.1. Mixed postsynaptic activation of GABAA/B -R and mGluR2 in GCs during high activity. ... 99
3.10.2. LLH implications in single cells and in the DG micro-circuit. ... 101
3.10.3. Delayed post-natal maturation of inhibitory circuits. ... 102
3.11. Supplemental Data ... 104
3.11.1. Application of a mGluR2 – specific antagonist ... 104
3.11.2. Application of mGluR2 and GABAB-R Positive Allosteric Modulators ... 106
Chapter 4. Synaptic recruitment of interneurons in the dentate gyrus molecular layer by the perforant pathway ... 114
4.1. Résumé ... 114
4.2. Abstract ... 115
4.3. Introduction ... 115
4.4. Interneurons in the molecular layer of the dentate gyrus show low excitability in response to PP stimulation. ... 118
4.5. Inhibition from local inhibitory network is not at the origin of low activation of Interneurons. ... 121
4.6. AP probability profiles of spiking ML-INs. ... 123
4.7. Similar Firing patterns of ML-INs correspond to distinct morphologies. ... 125
4.7. Long lasting hyperpolarization in molecular layer interneurons (ML-IN LLH). . 127
4.8. ML-IN LLH altered the cell’s firing pattern – AP distribution analysis. ... 129
4.9. ML-IN LLH is frequency dependent. Several features indicate that ML-IN LLH might be a frequency dependent mechanism. ... 131
4.10. ML-IN LLH is mediated via GABAA/B-Rs. ... 133
ix
4.11.1. Direct recruitment of ML-INs by the entorhinal terminals in the DG... 136
4.11.2. Differential firing patterns of PP recruited ML-INs. ... 137
4.11.3.LLH in interneurons of the molecular layer following PP stimulation ... 138
Chapter 5. On the Functional implications of slow inhibition as a fundamental component of sparse coding and transiently active assemblies’ generation ... 141
5.1. Summary ... 141
5.2. Short-term plasticity in information integration and memory ... 142
5.3. Long-lasting hyperpolarization (LLH) in the E/I balance ... 147
5.4. LLH potential in DG computations ... 148
5.5. Cooperativity of signaling pathways ... 149
5.6. What are the functional consequences of LLH alteration or removal? ... 150
5.7. Generation of transiently (in)active assemblies in the DG ... 151
5.8. Sparse activation of the interneuron network of the DG molecular layer ... 156
5.9. Slow inhibition in the interneuron network of the DG molecular layer ... 159
5.10. Slow inhibition in other hippocampal cells ... 160
5.11. LLH as a (slow) short-term inhibition with a post-synaptic origin ... 162
5.12. Functional implications at the circuit level ... 163
5.13. Future Directions ... 164
5.13.1. Exploring for links between LTP and LLH in GCs ... 164
5.13.2. Physiological EC pattern for “in vivo - like” stimulation ... 165
5.13.3. Dual channel optogenetics for activation or inhibition of different populations of interneurons ... 165
5.13.4. Large scale voltage sensitive dye (VSD) dynamics approach ... 166
5.13.5. Multiplex input effects on ML-IN recruitment. ... 167
5.13.6. FBI contribution for LLH. ... 167
x
5.14. Open questions ... 172
5.14.1. Functional connectivity classification. ... 172
5.14.2. Encoding vs Decoding in overlapping cell populations. Computational models and experimental data in hippocampal network theories. ... 174
5.14.3. Neural syntax... 177
5.14.4. Mechanisms of Neuronal allocation. ... 179
General Conclusion ... 181
xi
List of Figures
FIGURE 1.1.BIOFILMS PRODUCE SYNCHRONIZED OSCILLATIONS IN MEMBRANE POTENTIAL. ... 4
FIGURE 1.2.LEVELS OF NEURAL ORGANIZATION. ... 6
FIGURE 1.3.FIRST ILLUSTRATION OF CELLS OF THE NERVOUS SYSTEM BY CAJAL. ... 9
FIGURE 1.4.MULTIPLE DIMENSIONS OF INTERNEURON DIVERSITY. ... 12
FIGURE 1.5.DIFFERENT TYPES OF NEURONAL COMMUNICATION IN THE BRAIN. ... 14
FIGURE 1.6.ADAPTATION OF MICROBIAL OPSINS FROM NATURE FOR THE OPTICAL CONTROL OF NEURAL ACTIVITY. ... 17
FIGURE 1.7.DELIVERY OF OPSINS INTO THE MAMMALIAN BRAIN. ... 18
FIGURE 1.8.EVOLUTION OF THE HIPPOCAMPUS. ... 20
FIGURE 1.9.HIPPOCAMPAL STRUCTURE. ... 22
FIGURE 1.10.ORGANIZATION OF HIPPOCAMPUS PROPER LAYERS IN REFERENCE TO A PYRAMIDAL CELL. ... 23
FIGURE 1.11.PAGES FROM CLIVE WEARING'S DIARY. ... 27
FIGURE 1.12.PLACE CELLS... 30
FIGURE 1.13.GRID CELLS. ... 31
FIGURE 1.14.INNERVATION OF INTERNEURONS AND GRANULE CELLS IN THE MOLECULAR LAYER OF THE DENTATE GYRUS. ... 35
FIGURE 1.15.GRANULE CELL'S MORPHOLOGY. ... 38
FIGURE 1.16.GABAERGIC INNERVATION BY LOCAL INTERNEURONS IN THE DG. ... 44
FIGURE 1.17.METABOTROPIC GLUTAMATE RECEPTORS. ... 48
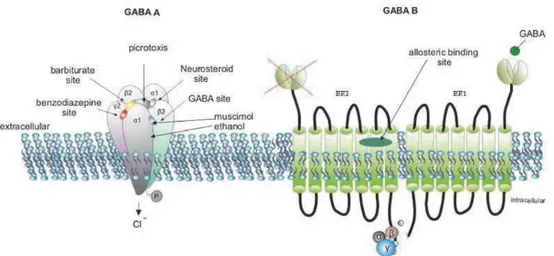
FIGURE 1.18.GABA RECEPTORS. ... 50
FIGURE 2.1.PAXINOS MOUSE BRAIN ATLAS MEC COORDINATES. ... 68
FIGURE 2.2.INCORRECT INJECTION SITE LEADS TO SPREAD OF THE VIRUS IN THE CA1 AREA OF THE HIPPOCAMPUS. ... 69
FIGURE 2.3.POSITION OF THE ENTORHINAL CORTEX. ... 70
FIGURE 2.4.ESTABLISHING MEC COORDINATES IN JUVENILE MICE. ... 70
FIGURE 2.5.EXPRESSION OF CHR2-MCHERRY IN THE MEC AND ALONG ITS PROJECTION IN THE DG. ... 71
FIGURE 3.1.EPSPS EVOKED BY PP STIMULATION ARE FOLLOWED BY A LONG-LASTING HYPERPOLARIZATION (LLH) IN DENTATE GCS. ... 78
FIGURE 3.2.LLH IS A MULTI-COMPONENT MECHANISM. ... 82
FIGURE 3.3.POSITIVE ALLOSTERIC MODULATORS (PAM) OF MGLUR2(LY487379) AND CGP7930... 85
FIGURE 3.4.LLH IS AN AGE-DEPENDENT MECHANISM. ... 87
FIGURE 3.5.LLH IS FREQUENCY-DEPENDENT. ... 89
FIGURE 3.6.LLH IS ACTIVITY-DEPENDENT. ... 90
FIGURE 3.7.LLH IS INDUCED THROUGH SYNAPTIC ACTIVATION FOLLOWING SPECIFIC PP STIMULATION. ... 92
FIGURE 3.8.NESTED LLH BURSTS ALTER AP FREQUENCY DISTRIBUTION DURING SINGLE INPUT STIMULATION. ... 95
FIGURE 3.9.SINGLE INPUT LLH CAN ALTER AP FREQUENCY DISTRIBUTION OF OTHER INPUTS. ... 98
FIGURE 3.10.THE MGLUR2 SPECIFIC ANTAGONIST LY341495 IN COMBINATION WITH GABAA/B-R ANTAGONIST BICUCULINE AND CGP55845. ... 105
FIGURE 3.11.LY487379(MGLUR2 POSITIVE ALLOSTERIC MODULATOR) DOES NOT SIGNIFICANTLY AFFECT LLH. ... 108
FIGURE 3.12.APPLICATION OF CGP7930(GABAB-R POSITIVE ALLOSTERIC MODULATOR) INCREASES LLH DURATION. ... 110
FIGURE 3.13.COMBINATION OF CGP7930 AND LY487379 AFFECTS LLH DURATION AND APFDT. ... 112
FIGURE 4.1.ML INTERNEURONS SHOW LOW EXCITABILITY IN RESPONSE TO PP STIMULATION. ... 120
FIGURE 4.2.BLOCKING INHIBITION DOES NOT AFFECT THE SHORT-TERM DEPRESSION OF PP INPUT IN THE MOLECULAR LAYER INTERNEURONS. ... 122
FIGURE 4.3.AP PROBABILITY PROFILES OF SPIKING ML-INS. ... 124
FIGURE 4.4.THREE GROUPS OF SPIKING ML-INS. ... 126
FIGURE 4.5.LONG-LASTING HYPERPOLARIZATION IN MOLECULAR LAYER INTERNEURONS (ML-INLLH). ... 128
xii
FIGURE 4.7.ML-INLLH IS FREQUENCY DEPENDENT. ... 132
FIGURE 4.8.ML-INLLH IS ENTIRELY MEDIATED VIA GABAA AND GABAB-RS. ... 134
FIGURE 5.1.GENERATION OF A MEMORY. ... 143
FIGURE 5.2.FUNCTIONAL ROLES OF SHORT-TERM PLASTICITY. ... 146
FIGURE 5.4.ENTORHINAL CORTEX INTERACTIONS IN THE MOLECULAR LAYER OF THE DG. ... 149
FIGURE 5.5.SUMMARY MODEL FOR THE GENERATION OF TRANSIENTLY ACTIVE ASSEMBLIES. ... 154
FIGURE 5.6.LLH IN CA3 PYRAMIDAL CELLS. ... 161
FIGURE 5.7.FEEDFORWARD INHIBITION IS SUFFICIENT FOR LLH EXPRESSION IN GCS. ... 169
FIGURE 5.8.ALTERNATING ACTIVATION OF EARLY, REGULAR, AND LATE SPIKING INTERNEURONS SHAPES THE LATE PHASE OF LLH. ... 170
FIGURE 5.9.LLH CAN BE INDUCED VIA DIRECT STIMULATION OF THE FBI. ... 171
FIGURE 5.10.ENCODING AND RETRIEVAL DURING DIFFERENT PHASES OF THE HIPPOCAMPAL THETA OSCILLATIONS ... 177
List of Tables
TABLE 1.GLUTAMATERGIC RECEPTORS. ... 45Abbreviations
AC Adenylyl Cyclase
ACSF Artificial Cerebro Spinal Fluid
AMPA-R α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
receptor
AP Action Potential
APF Action Potential Frequency
APFDT Action Potential Frequency Distribution in Time
BC Basket cell
BIC Bicuculine
CA1-3 "Cornus Ammonis" areas 1-3 in the hippocampus
Ca2+ Calcium ions
CAMKII Calmodulin-dependent protein kinase 2
CB1R Cannabinoid Receptors type 1
CCK Cholecystokinin (cholecystikinin expressing interneuron)
CCK-BC Choclecystokinin expressing Basket Cell
CED Convection Enhanced Delivery
ChR2 Channelrhodopsin-2
Cl- Chloride ions
CNS Central Nervous System
COM Commissural pathway
COM/AC Commissural/Associational pathway
COUP TFII COUP transcription factor 2
ctrl Control
DAG Diacyl- glycerol
DG Dentate Gyrus
E/I Excitation/Inhibition ratio
EC Entorhinal Cortex
EPSP Excitatory Postsynaptic Potential
FB Feedback
FF Feedforward
FFI Feedforward inhibition
GABA Gamma Aminobutyric Acid
GABAA/B -R GABA receptors type A and B
GABAA-R GABA Receptor type A
GABAB-R GABA Receptor type B
GABA-R GABA Receptors
xiv
GC Granule cell
GC-LLH Long-lasting Hyperpolarization in Granule cells
GEVI Genetically Encoded Voltage Indicators
GPCR G-protein coupled proteins
HEK Human Embryonic Kidney cells
HICAP Hilar-Commissural-Association Pathway cells
HIPP Hilar-Perforant-Pathway cells
IN Interneuron
IP3 inositol 1,4,5-trisphosphate
IPSP Inhibitiory Postsynaptic Potential
K+ Potassium ions
KA-R Kainate Receptor
Kir3 Inwardly Rectifying Potassium Channel
LEC Lateral Enthorhinal Cortex
LLH Long-lasting Hyperpolarization
LTD Long-term Depression
LTP Long-term Potentiation
MEC Medial Entorhinal Cortex
MEC II Projections of the Medial entorhinal cortex layer II
mGluR2 Metabotropic Glutamate Receptor type 2
mGluRs Metabotropic glutamate receptors
ML Molecular Layer
ML-IN Interneuron in the Molecular layer
ML-IN LLH Long-lasting Hyperpolarization in interneurons of the ML
MOPP Molecular-Perforant-Pathway associated cells
ms Milliseconds
NGFC Neurogliaform cell
NMDA-R N-methyl-D-aspartate Receptor
nNOS Neuronal Nitric Oxide Synthase
NPY Neuropeptide Y
PAM Positive Allosteric Modulators
PC Principal cell
PIP2 Phosphotinositides
PKA Protein kinase A
PKC Protein Kinase C, Protein kinase C
PN Principal Neuron
PP Perforant pathway
PSP Postsynaptic Potential
xv
PV-BC Parvalbumin expresing Basket Cell
SGC Semilunar Granule cells
SST Somatostatin (somatostation expressing interneuron)
STD Short-term Depression
STDP Spike-timing Dependent Plasticity
STP Short-term Potentiation
Sub Subiculum
SuM Supramammillary nucleus projections in the dentate gyrus
VIP Vasoactive Intestinal Polypeptide
xvi
“We are drowning in information,
while starving for wisdom.”
xvii
Acknowledgements
First and foremost, I would like to thank my supervisor Dr. Katalin Tóth. She gave me the great opportunity to fly across the ocean and do the science I was yearning to do since my very first class in Neuroscience. I am grateful for her patience, giving me the time and the space, I needed to grow as a scientist and as a person. Katalin taught me how to use data for what it is, using imperfection and deception as a power drive for creative thinking. I also have a great esteem for her capacity to detect and dissuade the overthinking I often leaned towards. Katalin is a master in putting things into perspective and I hope that I acquired this quality and that I would be able to apply it in my future endeavors. I must also admit that she is probably the only supervisor to send a student to “clean their mind at the pool” on a hot summer’s day, a facet of the Ph.D. life I was surely lucky and envied to experience. Undoubtedly, my scientific journey under her guidance proved to be also one of persistence, personal development, and growth.
I would like to thank my lab team for the technical and moral support over the years. I keep in my heart all the “broken fridge repairings" we attended together. I thank Simon Chamberland and Alesya Evstratova for teaching me the tricks of electrophysiology and Philippe Lemieux for never making PFA solutions for me; turns out it’s the best learning technique. My most heartfelt thanks go to Modesto Peralta for being the nice person everyone in the lab went to during difficult times. I will never forget all the weekends of surgery, mouse resuscitation and experimental troubleshooting. These of course, came along with many laughs and a great deal of random facts conversations he endured all these years. I can’t omit to thank him for keeping my spirits up by being my official chocolate provider. I am thankful to have him as a colleague but even more so, to have him as a friend.
I am grateful for the many great people I met in CERVO and who made the working environment so much better. I would like to thank our adopted team mate and lab neighbor – Aditi Sood, who often shared late lab evenings and Ph.D. wisdom with me. Special thanks to Theresa Wiesner for always being there for me and for all the weekend brunches; to Clementine Quintana for being the perfect partner in crime; to Kapil Sehgal for the encouragement and regular pep talks; to Lusine Bozoyan for her radiance and inspiring frame
xviii
of mind; to Archana Gengatharan for our “girl’s nights out” and risking to drive with me to explore Québec’s amazing nature; and to Louis Thibon for the fun game nights and all the French wine and cheese. I would also like to thank Sonya Bourgon for her help with all the administrative work.
Outside of the research center I feel lucky to have met Lise Lessard and Manon Lessard who became my Québec family.
I would like to thank my amazing Bulgarian friends, Tanya Pavlova and Gergana Tzelovska, for showing interest and appreciation of my scientific ventures and going through all my (not always solicited) monologues on “How does the brain work?”. Last, but not least, I thank my family for supporting all my decisions and helping me remain grounded all throughout this journey. I am extremely fortunate and grateful that our hearts have remained so close despite some 7,265 km and 7h between us. The knowledge of their unconditional love and understanding made this adventure possible.
xix
Foreword
This thesis was written as completion for a Ph.D. degree in Neuroscience accorded by Laval University. The work represents original research focusing on inhibition in the hippocampal dentate gyrus. The organization of this manuscript is as follows. Chapter 1 is an introduction into the scientific context of neuronal circuits. It is grossly divided into three parts, the first one being a short literary summary of what is known about the emergence of the nervous system. This part aims to acquaint the reader with the notion of integration of environmental stimuli in biological systems through neuronal communication. This part also includes an overview of the history and technological advances of the research tools that are used. The introduction continues with the detailed description of the hippocampus and further highlights the dentate gyrus as the region of interest. The accent is over synaptic plasticity and inhibitory interactions. The introductory chapter ends with the formulation of the objectives of this Ph.D. put in the framework of inhibition in the dentate gyrus.
Chapter 2 describes in detail the “Materials and Methods” that are used in the collection of the data.
Chapters 3 and 4 contain the results of the electrophysiological experiments carried by me during my Ph.D. training. While all experiments address interactions in the dentate gyrus, Chapter 3 looks upon the principal cells whereas Chapter 4 shifts the emphasis over the interneurons. The findings of Chapter 3 are the subject of a recent publication as a scientific paper where I am the first author. The results of Chapter 4 are currently being prepared for submission.
Chapter 5 proposes a general discussion of the results from three different angles. The first part provides a short summary of the findings and considers a direct interpretation of the data. The second part is presented as “Future Directions” and suggests several experimental designs designed to obtain a deeper understanding of the mechanisms and the functional implications of the inhibitory mechanism as the main finding of this manuscript. The third part offers a speculative analysis of the results in the light of modern ideas in neuronal communication. The manuscript ends with a “General Conclusion” section that revisits the main objectives of this thesis in direct association with the results.
1
General Introduction
The Darwinian evolution theory postulates that the principle of natural selection insures the survival of the fittest (Herbert Spencer, Principles of Biology, 1864) or “the preservation of
favored races in the struggle for life” (Darwin, 1859). To achieve this, it is programmed in
our primordial genetics to generate new variants of traits, to preserve those that insure an optimal adjustment, and ultimately - the continuity of the species. Following the principles of natural selection, the brain emerged and evolved as a coordinator of the external stimuli and the self-generated outcome in response to those stimuli.
The first signs of organized nervous system can be seen in some of the earliest fossils of the Precambrian Period (Kristan, 2016). The fossils reveal that the organisms of the period were mainly expressing passive feeding behaviors (suspension feeders such as foraminifera and brittle stars) and most of them were sessile (corals). These primitive life forms were presumably soft-bodied sponges, cnidarians and annelids. During the early Cambrian life explosion, diverse specialized features such as shells and spikes developed as defense mechanisms, suggesting an evolution towards predator-prey behavior. This evolution step was accompanied by a drastic increase in body size and compartmentalization of the biological functions. Active feeding behavior and massive physique now required the need of coordinated movements of the different parts of the upscaled body. This enabled the animals of that period to visualize a target, to plan an action and to perform directed movements towards their goal. In the same time the animals that become prey to others, they had to develop a whole other set of skills and mechanisms, such as faster movements and ability to sense danger, in order to escape the predators and to survive hostile environments. This implied subsecond integration of visual, sensory, and mechanical stimuli from the environment, and subsequently the equally fast and adequate generation of a behavioral output. This is how the ever-changing world selectively prioritized a system that is capable of ultra-fast processing of big blocks of information. Thus, it seems that fast integration of data was a driving power for brain development. The organisms endowed with such mechanism of multi-scale fast processing would have had the best chances to survive. The evolution of the modern mammalian nervous system underwent several major changes; it
2
centralized, grew bigger, developed a folding strategy of the neural tissue, and created specialized structures to treat different types of stimuli. It perceives, deconvolutes, integrates, and analyses information from the surrounding world and generates an output of signals that would in turn allow to plan and execute the appropriate reaction.
3
Chapter 1. Communication in the Neuronal
Circuits
1.1. Primitive communication and specialization of the neural cells
The diversity of stimuli drove the segregation of specialized groups of single units for reading and analyzing data representing different aspects of the environment. The smallest functional unit in the brain is the neuron. Diverse specializations define a multitude of cell types in the brain, each with differential contribution in brain development, maturation, learning and memory, sensory stimuli integration and behavior generation, motor coordination and life support life (e.g. breathing). The brain is constituted by millions of neurons and their main role is to maintain communication within a single network and coordination across the central nervous system. This is achieved by establishing multiple contacts with surrounding neurons or/and neurons located in neighboring brain areas. It is through these connections called synapses, that communication in the network is established. The excitability of the neuronal cell’s membrane is at the center of neuronal communication. Upon sufficient excitatory signals arriving via the synapses, the neuronal cell generates an impulse, called action potential (AP) which propagates down throughout the cell’s axonal projection. Neurons can receive and send information through chemical synapses via neurotransmitter release or electrical synapses and gap junctions. The increasing complexity of the organism’s functions favored a multi-layer neuronal organization of the central nervous system. Progressively, groups of cooperative neurons became functionally specialized and formed centralized brain areas. Through the linkage of neurons in neuronal networks, microcircuits emerged and interconnected all the areas of the brain. The development of a complex nervous system drove specializations at the subcellular level as well. The neuronal cell underwent changes in molecular mechanisms, allowing faster and efficient communication. The molecular building blocks for modern neuronal communication were already available even earlier than the first fossils found but lacked the efficiency of data processing that the modern brain needed. In fact, homologous genes for voltage gated channels and synaptic cleft molecules are found in bacteria (Figure 1.1), suggesting a high evolutionary conservation, a hallmark of essential genes (Kristan, 2016). Despite that the precise function of these molecular specializations in
4
earlier life forms is not known with certainty, it is suggested that voltage gated channels served solely to keep the integrity of unicellular organism membranes in water by regulating the intracellular ion concentrations. The need of fast synchronized communication became only evident when there were multiple cells that needed to act cooperatively. In this case, we can again take bacteria as an example of what could have been the functional benefits. When
a biofilm is formed - a grouping of many bacterial cells - the different cells use K+ ions release
as a signaling mechanism across the biofilm (Prindle et al., 2015). Bacterial ion channels
generate propagating waves of K+, creating a positive feedback loop which depolarizes
neighboring cells. These waves of depolarization are used to coordinate the metabolic state
of cells at the center and in the periphery of the biofilm (Figure 1.1). This communication
across the cells in a biofilm is called quorum sensing.
FIGURE 1.1.BIOFILMS PRODUCE SYNCHRONIZED OSCILLATIONS IN MEMBRANE POTENTIAL.
Prindle, A., Liu, J., Asally, M., Ly, S., Garcia-Ojalvo, J., & Süel, G. M. (2015). Ion channels enable electrical communication in bacterial communities. Nature, 527(7576), 59–63. https://doi.org/10.1038/nature15709
Figure 1.1. Original legend (Prindle et al., 2015): Biofilms produce synchronized oscillations in membrane potential. (A) Global oscillations of membrane potential reported
by thioflavin (ThT), within the biofilm community. ThT fluorescence is inversely related to the membrane potential. Scale bar, 0.15 mm; (B) An extracellular fluorescent chemical dye (APG-4) reports the concentration of potassium in the media. For comparison, the same cells are shown stained with ThT, which is inversely related to the membrane potential. Oscillations in membrane potential and extracellular potassium are synchronized, suggesting that potassium release is involved in global membrane potential oscillations. (C)
5
Illustration of the differences between passive signaling (diffusion) and active signaling. When cells passively respond to a signal, the range that the signal can propagate is limited due to the decay of signal amplitude. In contrast, when cells actively respond by amplifying the signal, propagation can extend over greater distances.
Although efficient in bacterial biofilms, quorum sensing through the diffusion of ions in water is slow and inefficient. Multicellular organisms needed to develop a faster method for activity synchronization. This is achieved by the emergence of the neural cell, specialized in the integration and transmission of information. The neuronal cell developed a long projection (axon) to connect surrounding cells and to insure a faster diffusion of signals through a very small space – the synaptic cleft. The emergence of neurons was followed by the segregation of multiple neuronal cells that connect to form a coordinated neuronal network. We consider the nervous system as the sum of organized and interconnected neuronal networks and their projections.
1.2. Modern brain specializations
The human brain contains ~ 100 billion neurons, more than 100 000 km of interconnections
and has an estimated storage of 1.25*1012 bytes (Cherniak, 1990; Hofman, 2012). This
massive communication network endows us with abstract thinking and cognitive capacity, and is presumed to be the siege of human consciousness. The origin of these complex and fascinating phenomena lies in the fast perception, analysis, and storage of multi-faceted information. This requires a high level of coordination across the nervous system components. The field of Neuroscience aims to elucidate how these operations are performed at subcellular, cellular, circuit and system levels, and ultimately how the brain integrates its
6
FIGURE 1.2.LEVELS OF NEURAL ORGANIZATION.
Figure 1.2: Levels of neural organization. To understand how the brain functions as a
system, neuroscientists investigate on every level of organization: brain areas, neural circuits, individual cells, and sub-cellular specializations.
1.3. Specialization of brain areas
Two main theories attempted to explain the origins of neuronal specialization: predetermined structural organization -the protomap view (Rakic, 1988), vs functional promiscuity – the protocortex view (O’Leary & Stanfield, 1989). The protomap theory correlates with a genetic program operating the neuronal fate and the formation of functional microcircuits, while the protocortex theory proposes an exclusively interactive strategy for the specialization of the neural circuits. Despite that both theories proposed interesting ideas to model the emergence of neural circuits, the exact strategy by which those gradually emerged remains not fully understood. However, the ontogenetic approach has allowed for the collection of convincing data in favor of the protomap view. It is now well established that a wide range of genetically programmed mechanisms contribute to cell organization in functional cortical maps in early neurogenesis: secreted molecules for neuronal guidance, morphogenetic signals, spontaneous embryonic activity, prenatal calcium waves. Altogether, this contributes to the patterned distribution of neurons across the neocortex (Bishop et al., 2000; Mallamaci et al., 2000; Fukuchi -Shimogori et al., 2001; Antón-Bolaños et al, 2019; Botella-López et al., 2019). While the protomap theory provides evidence for the emergence of specialized neural cells and networks, the protocortex view proposes a hypothesis of how these networks support lifelong memory storage, the ability to learn new skills, and acquire new information. Although we know that neurons differentiate and integrate circuits following a predetermined
7
genetic program, the question of how do we learn and remember, remained unexplained by the protomap view. Indeed, further studies of neuronal circuits demonstrate that the protocortex view was rather accurate in terms of neuronal interactions at the origins of the ability to acquire new memories and skills. It is now well established that learning is achieved via dynamic synaptic plasticity changes in neuronal networks in response to environmental stimuli.
1.4. Specialization of the neural networks
The molecular identity and location that the neuronal cell will occupy in the cortical circuits is determined during neurogenesis. Through secreted molecular guidance compounds, the cell migrates to reach its target region, establish synaptic connections with the surrounding cells and differentiates into a mature neuron. This process takes place early in development; by the time of the birth, most of the neurons are already present and/or undergoing significant maturation processes. Early postnatal development includes synaptogenesis, pruning, inhibitory mechanism maturation, GABA conversion to hyperpolarizing signals, synaptic strengthening, and spatio-temporal precision of coordinated activity in functional networks through new experiences (Kolb & Whishaw, 2003). Thus, the cortical circuits are highly flexible in the early post-natal development in order to reach an optimal efficiency of information processing. The synaptic flexibility decreases into adulthood, nevertheless remains important throughout adult life. Some of the main plasticity mechanisms are described further (1.8.3 Synaptic plasticity) in the framework of synaptic communication in the hippocampal dentate gyrus.
1.5. Study of the neural circuits
1.5.1. Emergence of Neuroscience
The growing complexity of the nervous system was at the origin of adaptive behavior, coordinated movement, emergence of vision, learning and memory storage. In correlation, the gradual emergence of neural circuitry architecture is the result of these functions. It is interesting why such complex system with high energy costs (Aiello & Wheeler, 1995) has been preserved through the natural selection. The only way to obtain a better understanding is to elucidate how single cells are functionally relevant to the circuits, and furthermore, the rules that govern the integration of different neural circuits in the whole nervous system.
8
These problems are currently being addressed through a structural mapping of the brain, and studying the functional connections at all the levels: subcellular, cellular, circuit and system. Modern neuroscience relies on several advanced techniques to endeavor that knowledge.
1.5.2. Neuroanatomical studies and early classification system of neuronal cell
types
The history of neuronal “discovery” starts with the first observations of animal and human tissue (Malpighi, 1666; Gennari, 1782; see Jones, 1984 for detailed review). Drawings based on these studies already started to raise the idea of neuronal cells as individual functional units (functioning in continuum or individually). Although the cellular structure was not yet entirely visible, at least two different cell types were commonly recognized: pyramidal and nonpyramidal (Jones, 1984). With the development of neural staining (Von Gerlach, 1872; Golgi, 1873) the complete structure of the first cortical neurons became available. The
existence of different cell types comes from the works of Santiago Ramón y Cajal (Figure
1.3; Cajal, 1890). He used the Golgi method (Golgi, 1873) to stain single neurons in fixed
brain tissue, which led to multiple studies with illustrations of neural cell morphologies. The work of Golgi and Cajal was followed by a series of observations in brain areas, both in animals and humans. With the growing number of identified neuronal cells in each study, the need of a nomenclature and classification became evident. Since the available techniques allowed for the visual characterization, most often the morphology was used as classification criteria – location and shape of the soma (pyramidal cells), dendritic arbor architecture (spiny/aspiny), projections (short-axon cells). The morphology of the cells was also thought to reflect their connectivity (Lorente de Nó, 1933) in the “neuronal forest”. Thus, beyond the visual description, the classification of neurons aimed to deduce common functions based on shared characteristics and, allowed for speculation of the relationships between different classes of cells. Morphological classification is thus the first attempt of a functional classification.
9
FIGURE 1.3.FIRST ILLUSTRATION OF CELLS OF THE NERVOUS SYSTEM BY CAJAL.
Javier DeFelipe, Cajal and the discovery of a new artistic world: The neuronal forest, 2013; Changing Views of Cajal’s Neuron, Chapter 8
Figure 1.3: First illustration of cells of the nervous system by Cajal (Cajal, 1888). Original
legend: “Vertical section of a cerebellar convolution of a hen. Impregnation by the Golgi method. A, represents the molecular zone, B, designates the granular layer, and C the white matter.”.
Early nomenclature proposed by Cajal describes long-projections (principal cells) and “short-axon” cell that we consider today as equivalent to interneurons (DeFelipe, 2002). This classification originates from the local interneurons that tend to synapse with the surrounding cells from the same area. These cells are particularly interesting as they present the widest range of diverse morphologies across multiple brain structures. The “short-axon” interneurons were thought to function as relay between long-projection neurons. Cajal (Cajal, 1901-1902) states that discharges of the interneurons would contribute to the multiplication of the signals of the long-projection neurons. It is not until the 1960s that the inhibitory roles of interneurons became a popular idea, and was further confirmed using new techniques such as immunochemistry (DeFelipe, 2002). Cajal was also the first one to notice the great diversity of this class, such as he didn’t attempt to group them in different types, but defined two main classes based on the available morphological criteria: Golgi cells and Martinotti cells.
With the development of new techniques for the study of neuronal networks, new criteria became also available and allowed for a more systematic classification. Pyramidal cells and interneurons from the same class would have similar effects over their postsynaptic targets.
10
Although principal cells are far more numerous than interneurons (Woodson et al, 1989; Bezaire & Soltesz, 2013), interneurons are the most diverse neuronal population in the central nervous system with probably around hundred distinct types described (Parra et al., 1998; Markram et al., 2004). Eventually, the identification of a cell type should give a prediction on their role in the neuronal circuit. As we continue to discover more neuronal cell features (function, physiology, molecular markers, etc.) the classification process resembles an hourglass cell sorting paradigm; the result of which is a growing number of types of interneurons.
1.5.3. Functional Study of the neuron
The excitability of their membrane enabled the functional studies of the neurons. The differential concentration of ions between the extracellular and intracellular space generates a membrane potential. The movement of ions through the neuronal cell membrane allows fluctuations in the resting membrane potential and the generation of action potentials (AP) upon binding of a neurotransmitter substrate to its postsynaptic receptor. The AP is accompanied by the release of neurotransmitter molecules in the synaptic cleft. The AP propagation across a neural network creates waves of excitatory activity and allows for neural communication which is at the origin of data processing (Hille, 1991). Initially, slow metabotropic channels were efficient enough for the early organisms that were sessile, passive feeders. However, as described earlier, this method became highly inefficient with the development of more complex behaviors. Communication through AP propagation and ionotropic channels ensures faster processing of information, a skill that has been preserved by natural selection.
After the initial observations that electric stimulation provokes muscle contractions (Galvani, 1791; Aldini, 1804; Nobili, 1828; Matteucci, 1842; du Bois-Reymond, 1884; Cobb, 2002), refinement of the stimulation techniques, discovering ways for time-precise recordings of the elicited responses, and ways to measure the electric propagation, all converged in the establishment of the electrophysiology method. The voltage clamp was developed in the 1940s (Curtis & Cole, 1938.; Marmont, 1949) giving a proof for nerve pulse propagation in the giant squid axon. The results of following studies using electrophysiology recordings allowed for the formulation of the Hodgkin-Huxley model (Hodgkin & Huxley, 1952) of
11
action potential generation. The model is based on the increased conductance of Na+ to
generate the action potential followed by increase in K+ permeability to facilitate a fast return
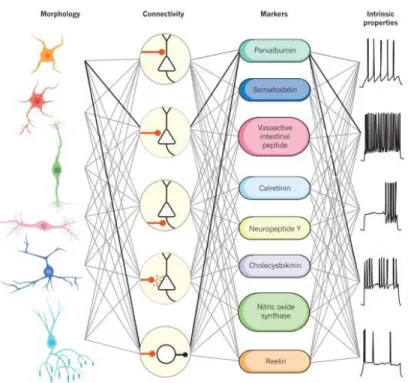
to resting membrane potential. The patch-clamp technique was developed in the 1970s (Neher & Sakmann, 1976) which opened the door to study of the ion channel properties and signal-transduction mechanisms (Verkhratsky et al., 2006). Furthermore, new methods to prepare in vivo probes of different nervous tissues, including mammalian brains and where the neurons can be kept alive over several hours, were also developed. The electrophysiology underwent numerous technical refinements and became a classic method to measure the activity of single neurons in the network. Most importantly, the intense study of the electric properties of neurons allowed for an increased interest towards the question of how the neurons communicate in a coordinated manner in the central and peripheral nervous system. This increased interest for the functional properties of neuronal networks resulted in the invention of the patch-clamp technique (Neher & Sakmann, 1976; Hamill et al., 1981). This new technological advancement allowed for a more detailed knowledge about synaptic transmission and membrane properties during information processing. Using patch-clamp, one can measure ionic currents in isolated live cells and communication between pairs of connected neurons. Moreover, this technique presently allows for the simultaneous electrophysiological recordings from single neurons in vivo, in anesthetized and in awake, behaving animals. We can collect online recordings of neurons encoding environmental cues and generating a behavioral output in model animals. In combination with the anatomical identification of neuronal populations, differential neuronal types, the functional study shows that interneurons can also exhibit different physiological profiles (Figure 1.4). Since the electrophysiology method offers an insight into neuronal communication, the completion of the functional brain map has become one of the main goals in Neuroscience.
12
FIGURE 1.4.MULTIPLE DIMENSIONS OF INTERNEURON DIVERSITY.
Kepecs, A., & Fishell, G. (2014). Interneuron cell types are fit to function. Nature, 505(7483), 318–326. https://doi.org/10.1038/nature12983
Figure 1.4: Original legend: Multiple dimensions of interneuron diversity. Interneuron cell
types are usually defined using a combination of criteria based on morphology, connectivity pattern, synaptic properties, marker expression and intrinsic firing properties (Kepecs & Fishell, 2014).
1.5.4. Communication in the neuronal networks and the Excitation/inhibition
Balance
Neuronal communication in the network is achieved by electrical or chemical signaling. Electrical signaling can take place through specialized structures called gap junctions that allow for the direct flow of electrical currents between two cells. Alternatively, in absence of such specialized structures that enable the contact between the cells, ephaptic interactions, generated by the extracellular electric field as the result of electrical activity of neurons, also occur. For this communication mechanism, the current associated with a presynaptic action potential is imposed on the postsynaptic membrane because of the high extacellular impedance (Weiss & Faber, 2010). In electrical coupling there is no delay between the
13
presynaptic AP and the postsynaptic field effect. Whether this effect would be excitatory or inhibitory would depend on the direction and the power of the current flow at the postsynaptic site. Chemical communication is established through specialized connections called synapses. It is realized by the release of neurochemical molecules upon AP emission from the presynaptic neuron in the synaptic cleft and the receptor-specific binding at the postsynaptic membrane. Receptors on the postsynaptic membrane represent a ligand-gated ion channels that generate electrical signal upon the binding of the neurotransmitter. Whether the postsynaptic response would be excitatory or inhibitory depends on the ion permeability of the receptor. Another form of chemical communication is volume transmission, based on the diffusion of neurotransmitters from one cell to many of the surrounding neighboring cells without specific synaptic contact.
Electrical transmission ensures faster signaling between neurons; however, it lacks mechanisms for amplification and transformation of the signals (Bennett & Zukin, 2004). Electrical transmission through gap junctions can be a powerful communication method particularly for synchronization of the activity of group of neurons. They play important roles for the rhythmicity and the synchrony of local field potentials in the hippocampus (Weiss & Faber, 2010). Volume transmission has a one-to-many, non-target specific signaling that occurs at a slower time scale (Agnatti et al., 2010). Thus, volume transmission is important for the summation of slower changes that take place over larger areas (Taber & Hurley, 2014). The spatio-temporal precision and the selectivity of information integration is best
attained by the chemical neurotransmission (Figure 1.5). The timing and the frequency of the
action potential generation is considered as the two main communication codes in neural circuits (Adrian, 1928; Rieke et al., 1997; Dayan & Abbott, 2001).
14
FIGURE 1.5.DIFFERENT TYPES OF NEURONAL COMMUNICATION IN THE BRAIN.
Figure 1.5. Different types of neuronal communication in the brain (original table from
Agnatti et al. 2010).
Chemical neurotransmission operates via excitatory and inhibitory signals at the synapse. In general principal cells in the circuits are excitatory and the local interneurons provide inhibition. However, there are several notable exceptions such the Purkinje cells (Ito & Yoshida, 1966) in the cortex of the cerebellum and the intercalated cells in the amygdala (Bienvenu et al., 2015). Long-range inhibitory projections also connect different brain regions (Melzer et al., 2012) and can send both excitatory or inhibitory cues. Excitatory signals would cause the postsynaptic cell to depolarize via the opening of ion channels and the activation of secondary intracellular messengers. Inhibitory signals on the other hand will in general cause the cell’s hyperpolarization, driving it away from the action potential threshold. In addition to inhibitory events that result in a noticeable postsynaptic IPSP, inhibition can also occur without obvious change of the membrane potential. Shunting inhibition causes an increase in synaptic conductance that is sufficient to interfere with excitatory currents generated at adjacent synapses (Jonas & Buzsaki, 2007). The increased synaptic conductance can counteract the membrane depolarization by decreasing its effect over the voltage-gated channels at the post-synaptic site (Destexhe et al. 2003).
The excitation/inhibition (E/I) ratio is maintained at balance during basal network activity but undergoes dynamic alterations during different behavioral states (Fernandez et al., 2007; Kehrer et al., 2008; Gogolla et al., 2009). The synchronization of activity is also important for the coordination of signals in time and space across the network and different brain areas.
15
Additional modulations of neurotransmission can be achieved via neuromodulators such as dopamine, serotonin, acetylcholine etc. (Hille, 1991). These alterations of the E/I ratio represent the synaptic plasticity required for information processing.
1.6. Mapping the brain circuits – combined techniques for system
neuroscience studies
Brain mapping is defined “as the study of the anatomy and the function of the brain and
spinal cord through the use of imaging, immunohistochemistry, molecular and optogenetics, stem cell and cellular biology, engineering, neurophysiology and nanotechnology” (as
defined by Society for Brain Mapping and Therapeutics, 2013). The interdisciplinary character of modern neuroscience allows to build a better understanding of different aspects of the circuitry of neurons and the role of single cells in the circuits. The constant development and refinement of new ways to study the brain, allow for the better description and understanding of the connectome and provides a variety of tools to manipulate the neural circuits. Electrophysiology is the main experimental approach involved in the data collection presented in Chapters 3 and 4 along with the following techniques, briefly presented below.
1.6.1. Pharmacological approach
Neuroscience is a highly interdisciplinary field. Biologists, pharmacologists, physicians, engineers, chemists, physicists, mathematicians, psychologists, social scientists, data scientists, and computer science developers are all interested in different aspects of how the brain is constructed, how it works, and how we can learn to develop better health care, improve quality of life, understand the seat of consciousness and build better machines. In fundamental cellular and molecular neuroscience research we profit from the tools developed in molecular neuropharmacology. This field develops molecules that can target specific neuronal properties and investigates neuronal network functions via the interactions evoked by the application of the neurochemical compounds. A big part of the molecules targets the relationship between neurotransmitter release from the presynaptic neurons and its binding to receptors on the postsynaptic membrane. The interaction with the compound can be either disrupting (competitive and non-competitive antagonists), enhancing (agonists), or modulatory (positive/negative allosteric modulators). Using receptor-specific
16
pharmacological molecules, electrophysiologists obtain some degree of control over the neural network and can probe the functions of isolated receptors.
1.6.2. Optogenetics
The combination of electrophysiological and pharmacological approaches is extremely useful. Thanks to this powerful combination, we have obtained extensive knowledge of the electrogenic properties of the neurons, the expression of specific receptors at the surface of the membrane and their functional consequences, both at single cell level, and at circuit level. However, it does not allow for the selective manipulation of a specific type of neurons and the assessment of functions in the circuits. Application of electrical stimulation via glass pipette in brain slices activates more than one terminal and targeted single cell stimulation in paired recordings is a time-consuming and low efficiency method. Precise spatio-temporal control over the neural networks is achieved by the development of the optogenetics method. Optogenetics represents a wide range of tools, that is constantly under the development of refining techniques to tackle neural functions. The optogenetic tools are genetically encoded, light-sensitive channels. They can be expressed in specific target neurons via viral vectors, where light stimuli can activate them. The activation of the light-sensitive channel drives the depolarization and the action potential generation (channelrhodopsin-2) of the cell or a hyperpolarization (halorhodopsin).
The first opsins were discovered in the 1970s (Oesterhelt & Stoeckenius, 1971; Oesterhelt & Stoeckenius, 1973), as natural occurring in bacteria, light-driven ion pumps. They are seven - transmembrane domains proteins, that can move ions across the membrane when illuminated (Boyden, 2011). Initial studies of opsins were motivated by their transformation of light into energy source and a sensory signal. Over a couple of years, green-light sensitive (archaeon) channel that moves protons out of the cell and orange-light sensitive halorhodopsine that causes an inward current of Cl- were discovered expressed in
Halobacterium salinarum (Oesterhelt & Stoeckenius, 1971; Oesterhelt & Stoeckenius,
1973). Following the publication of these discoveries, neuroscientists became highly interested in the potential of opsins to be used to tackle neuron’s functions in the brain circuits. This interest for the opsin’s potential as a tool resulted in 2003 in the discovery that the channelrhodopsin-2 (ChR2), a green- algae opsin, (Sineshchekov et al., 2002) can be used to depolarize cells by importing protons in oocytes and HEK cells upon light stimulation
17
(Nagel et al., 2003). The authors then predicted that channelrhodpsin-2 would be a valuable
tool for manipulation of intracellular Ca2+ concentration in mammalian cells. Just a couple
of years later, Boyden et al., (Boyden et al., 2005) published a paper proving that ChR2 can be successfully transfected in neurons. Moreover, upon light stimulation, the expressing neurons were repeatedly activated proving the functionality of the channelrhodpsin in
neurons (Figure 1.6). During the same time, other groups pioneered the optogenetics field by
expressing functional opsins to light driven activity in C. elegans (Nagel et al., 2005) and in
Drosophila (Zemelman et al., 2002). The application of optogenetics rapidly outgrew these
initial studies and is it is now widely used to tackle all sort of brain circuitry questions. A significant advance for the study of neurons as part of communication network is that the expression of optogenetic molecules provides fast and precise stimulation. This type of light-driven stimulation can be also made population specific (ex. targeting via specific Cre-recombinase expression) or expressed under a specific promoter/enhancer (ex. CAMKII in excitatory cells). Optogenetic tools are also very versatile and compatible with many other techniques like imaging, electrophysiological recordings in vitro and in vivo, behavioral paradigms, optotagging (photostimulation-assisted identification of neuronal populations).
FIGURE 1.6.ADAPTATION OF MICROBIAL OPSINS FROM NATURE FOR THE OPTICAL CONTROL OF NEURAL ACTIVITY.
E. Boyden, A history of optogenetics: the development of tools for controlling brain circuits with light, F1000 Biology Reports, 2011
Figure 1.6: Original legend: Adaptation of Microbial opsinsfrom nature for the optical control of neural activity. (A-C) Diagrams depicting the physiological responses of (A)
archaerhodopsins and bacteriorhodopsins (light-driven outward proton pumps), (B) halorhodopsins driven inward chloride pumps), and (C) channelrhodopsins
(light-18
gated inward nonspecific cation channels), when expressed in the plasma membranes of neurons and exposed to light (Boyden, 2011).
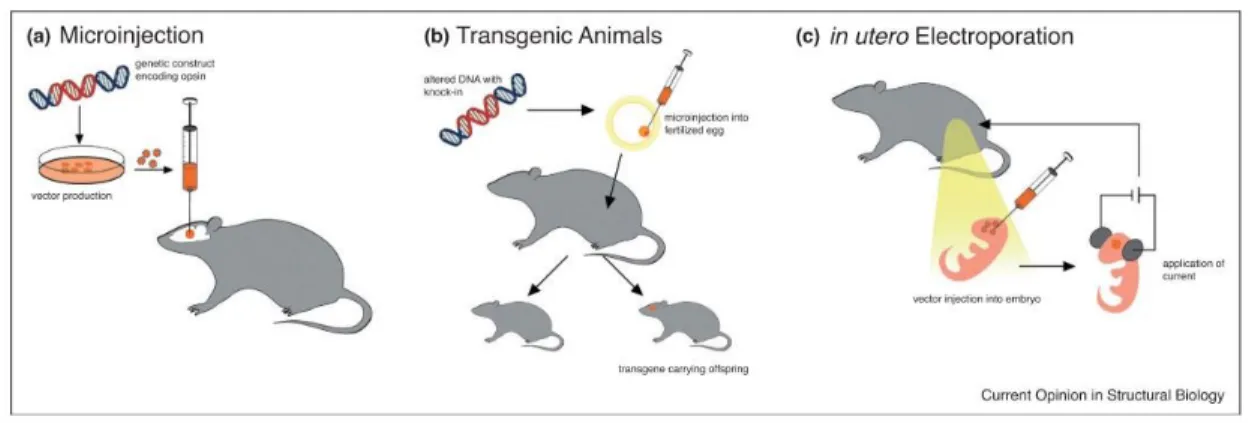
The expression of ChR2 in the brain can be achieved by viral vector delivery in young/adult animal models (C. elegans, Drosophila, zebrafish, mouse, rat, and nonhuman primate) and
direct intracranial injection, by transgenic modifications or in utero electroporation (Figure
1.7). In in vitro preparations of ChR2 can be activated using mercury or xenon lamp, lasers,
or diodes. For in vivo experiments an optical fiber can be permanently implanted in head fixed or freely moving animals.
FIGURE 1.7.DELIVERY OF OPSINS INTO THE MAMMALIAN BRAIN.
Jan Deubner, Philippe Coulo and Ilka Diester, Optogenetic approaches to study the mammalian brain, Current Opinion in Structural Biology, 2019
Figure 1.7: Original legend (Deubner et al., 2019):Delivery of opsins into the mammalian brain. (a) A genetic construct carrying opsin genes and other elements (promoters etc.) are
packaged in viral vectors which are microinjected directly into the brain. This can further be refined by convection enhanced delivery (CED) or combined with targeting strategies to achieve cell type specificity. (b) Altered genetic material (e.g. knock-in) is injected into fertilized eggs and implanted into pseudo-pregnant females to create transgenic animals. (c) To enable investigations of the developing brain, a solution with opsin containing DNA is injected into embryo brains and specifically directed to certain cell subpopulations by electroporation.
Optogenetics quickly revolutionized neuroscience research by allowing for the targeted circuit investigation. These tools are now broadly used in brain mapping (Petreanu et al.,
19
2007; Atasoy et al., 2008; Kuhlman & Huang, 2008; Kohara et al., 2014; Yamamoto & Tonegawa, 2017), assessing neuronal functions in a circuit during behavior (Zhang et al., 2007; Huber et al., 2008; Douglass et al., 2008;), sensorimotor (Hägglund et al, 2010), pain mechanisms (Samineni et al., 2017a,b; Tashima et al., 2018; Sugiyama et al., 2019), vision (Farah et al., 2007; Lagali et al., 2008), breathing (Alilain et al., 2008), motor functions (Aravanis et al., 2007), fear (Klavir et al., 2017), and has been useful in cardiac physiology (Sasse et al., 2019). Optogenetic methods also help study pathogenesis and the therapeutic strategies in epilepsy (Krook-Magnuson et al, 2014), Parkinson disease (Magno et al., 2019) and chronic pain (Zhang et al., 2017).
1.7. Hippocampus – highlight over the Dentate gyrus
1.7.1. Origin and evolution of the hippocampus
The hippocampal structure is well conserved among vertebrates and has a long evolutionary history. The emergence and the evolution of the hippocampus is not entirely elucidated and there is a lack of data on the functional and neurobiological features of the nonmammalian hippocampus. However, this subject is under intensive investigation and Bingman and Muzio (Bingman & Muzio, 2017) propose the hypothesis that the hippocampal formation in modern amniotes developed from a medial pallium structure (a structure in birds and fish considered
homologous to the mammalian hippocampus) (Figure 1.8). The primitive pallium had
undifferentiated organization and had a major role in learning and memory processes that required spatial reference. This relatively simple construction served as a base for the more structured and specialized hippocampal areas that arose in birds and mammals. A highlight of the evolution of the mammalian hippocampus is the dentate gyrus (Hevner, 2016). The dentate gyrus is considered a part of the archicortex (allocortex or also non-neocortex) based on the similarity to the reptilian medial cortex (Ariëns Kappers et al., 1936–1965; Carpenter, 1976; Striedter, 2005). The allocortex of mammals arose from the reptilian dorsal cortex and surrounds the neocortex (Puelles, 2001, 2011; Medina & Abellán, 2009). Although homologous structures of the DG are identified in reptiles (Marchioro et al., 2005) and in avian medial pallium (Gupta et al., 2012; Abellán et al., 2014), in mammals this structure is larger, convoluted and is an adult neurogenesis niche (Treves et al., 2008; Kempermann, 2012; Aimone et al., 2014; Christian et al., 2014).
20
The hippocampus was first anatomically described as a silkworm or seahorse shaped brain structure by the Venetian anatomist Julius Caesar Aranzi in 1587. It is eventually the Greek word for seahorse, hippocampus, that became the common term designating this structure (Duvernoy, 2005). The term “cornus ammonis”, was first mentioned by the French surgeon René-Jacques Croissant de Garengeot in 1742 to designate the different areas of the
hippocampus (Duvernoy, 2005).Today we use the abbreviation CA (CA1-4) to refer to these
areas.
FIGURE 1.8.EVOLUTION OF THE HIPPOCAMPUS.
Bingman and Muzio, 2017 (Bingman VP, Muzio RN. Reflections on the Structural-Functional Evolution of the Hippocampus: What Is the Big Deal about a Dentate Gyrus? Brain Behav Evol. 2017; 90(1):53-61. doi: 10.1159/000475592. Epub 2017 Sep 4. PubMed PMID: 28866681.).
Figure 1.8: The Evolutionary reconstruction of the evolutionary path of the hippocampus proposed by Bingman and Muzio, 2017. The figure describes the evolution from ancestral medial pallium of an amphibious stem tetrapod (telencephalon coronal section, Nissl stained, African clawed frog (Xenopus laevis); A, amygdala; DP, dorsal pallium; LP, lateral pallium; MP, medial pallium; Sep, septum; Str, striatum) to the hippocampal formation of birds (Nissl stained coronal section from the telecenephalon of a homing pigeon (Columba livia); Hp,