Accepted Manuscript
The dementia-associated APOE ε4 allele is not associated with REM sleep behavior disorder
Ziv Gan-Or, MD, PhD, Jacques Y. Montplaisir, MD, PhD, Jay P. Ross, BSc, Judes Poirier, PhD, Simon C. Warby, PhD, Isabelle Arnulf, MD, PhD, Stephanie Strong, BSc, Yves Dauvilliers, MD, PhD, Claire S. Leblond, PhD, Michele T.M. Hu, MBBS, FRCP, PhD, Birgit Högl, MD, Ambra Stefani, MD, Christelle Charley Monaca, MD, PhD, Valérie Cochen De Cock, MD, PhD, Michel Boivin, PhD, Luigi Ferini-Strambi, MD, PhD, Giuseppe Plazzi, MD, PhD, Elena Antelmi, MD, Peter Young, MD, Anna Heidbreder, MD, Thomas R. Barber, MA, MBBS, MRCP, Samuel G. Evetts, BSc Hons, MSc, Michal Rolinski, BM BCh, BA Hons, MRCP, Patrick A. Dion, PhD, Alex Desautels, MD, PhD, Jean-François Gagnon, PhD, Nicolas Dupré, MD, MSc, Ronald B. Postuma, MD, MSc, Guy A. Rouleau, MD, PhD
PII: S0197-4580(16)30242-1
DOI: 10.1016/j.neurobiolaging.2016.10.002 Reference: NBA 9741
To appear in: Neurobiology of Aging Received Date: 18 August 2016 Revised Date: 22 August 2016 Accepted Date: 1 October 2016
Please cite this article as: Gan-Or, Z., Montplaisir, J.Y., Ross, J.P., Poirier, J., Warby, S.C., Arnulf, I., Strong, S., Dauvilliers, Y., Leblond, C.S., Hu, M.T.M., Högl, B., Stefani, A., Monaca, C.C., De Cock, V.C., Boivin, M., Ferini-Strambi, L., Plazzi, G., Antelmi, E., Young, P., Heidbreder, A., Barber, T.R, Evetts, S.G., Rolinski, M., Dion, P.A., Desautels, A., Gagnon, J.-F., Dupré, N., Postuma, R.B., Rouleau, G.A., The dementia-associated APOE ε4 allele is not associated with REM sleep behavior disorder, Neurobiology of Aging (2016), doi: 10.1016/j.neurobiolaging.2016.10.002.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please
note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
M
AN
US
CR
IP
T
AC
CE
PT
ED
ACCEPTED MANUSCRIPT
1The dementia-associated APOE εεεε4 allele is not associated with REM sleep behavior disorder
Ziv Gan-Or, MD, PhD,a,b,c, Jacques Y. Montplaisir, MD, PhD,d,e, Jay P. Ross, BSc,b Judes Poirier, PhD
f,g,
Simon C. Warby, PhD,4,5, Isabelle Arnulf, MD, PhD,h, Stephanie Strong, BSc,a, Yves Dauvilliers,
MD, PhD,i, Claire S. Leblond, PhD,1,b, Michele T.M. Hu,MBBS, FRCP, PhD,j,k, Birgit Högl, MD,l,
Ambra Stefani, MD,l, Christelle Charley Monaca, MD, PhD,m, Valérie Cochen De Cock, MD, PhD,n,o,
Michel Boivin, PhD,p,q,Luigi Ferini-Strambi, MD, PhD,r, Giuseppe Plazzi, MD, PhD,s,t, Elena Antelmi,
MD,s, Peter Young, MD,u, Anna Heidbreder, MD,u,Thomas R Barber MA, MBBS, MRCP,j,k, Samuel G.
Evetts BSc Hons, MSc,j,k, Michal Rolinski, BM BCh, BA Hons, MRCP,j,k, Patrick A. Dion, PhD,a,c,
Alex Desautels, MD, PhD,d,v, Jean-François Gagnon, PhD,d,w, Nicolas Dupré, MD, MSc,x, Ronald B.
Postuma, MD, MSc,c,y, and Guy A. Rouleau, MD, PhD,a,b,c.
Affiliations : a
Montreal Neurological Institute, McGill University, Montréal, QC,H3A 0G4, Canada, bDepartment of
Human Genetics, McGill University, H3A 0G4, Montréal, QC, Canada, cDepartment of Neurology and
neurosurgery, McGill University, Montréal, QC, H3A 0G4, Canada, dCentre d’Études Avancées en
Médecine du Sommeil, Hôpital du Sacré-Cœur de Montréal, Montréal, QC, H4J 1C5, Canada,
e
Department of Psychiatry, Université de Montréal, Montréal, QC, H3T 1J4, Canada, fDepartment of
Psychiatry, McGill University, Montréal, QC, H3A 0G4, Canada, gDouglas Mental Health University
Institute, Montréal, QC, H4H 1R3, Canada, hSleep Disorders Unit, Pitié Salpêtrière Hospital, Centre de
Recherche de l’Institut du Cerveau et de la Moelle Epinière and Sorbonne Universities, UPMC Paris 6
univ, Paris, 75013, France, iSleep Unit, National Reference Network for Narcolepsy, Department of
Neurology Hôpital-Gui-de Chauliac, CHU Montpellier, INSERM U1061, Montpellier, 34000, France, j
Oxford Parkinson’s Disease Centre (OPDC), University of Oxford, Oxford, OX1 2JD, United
Kingdom, kNuffield Department of Clinical Neurosciences, University of Oxford, Oxford, OX1 2JD,
United Kingdom, lSleep Disorders Clinic, Department of Neurology, Medical University of Innsbruck,
Innsbruck,6020, Austria, mUniversity Lille north of France, Department of clinical neurophysiology and
sleep center, CHU Lille, Lille, 59000, France, nSleep and neurology unit, Beau Soleil Clinic,
Montpellier, 34070, France, oEuroMov, University of Montpellier, Montpellier, 34095, France, pGRIP,
École de psychologie, Université Laval, Québec city, QC, G1V 0A6, Canada, qInstitute of Genetic,
Neurobiological and Social Foundations of Child Development, Tomsk State University, Tomsk,
634050, Russia, rDepartment of Neurological Sciences, Università Vita-Salute San Raffaele, Milan,
20132, Italy, sDepartment of Biomedical and Neuromotor Sciences (DIBINEM), Alma Mater
Studiorum, University of Bologna, Bologna,40126, Italy, tIRCCS, Institute of Neurological Sciences of
Bologna, Bologna, 40139, Italy, uDepartment of Sleep Medicine and Neuromuscular Disorders,
University of Muenster, 48149, Germany, vDepartment of Neurosciences, Université de Montréal,
Montréal, H3T 1J4, Canada, wDépartement de psychologie, Université du Québec à Montréal, Montréal,
QC, H2L 2C4, Canada, xFaculté de Médecine, Université Laval, CHU de Québec (Enfant-Jésus),
Québec, QC, G1J 1Z4, Canada, yDepartment of Neurology, Montreal General Hospital, Montréal, QC,
M
AN
US
CR
IP
T
AC
CE
PT
ED
ACCEPTED MANUSCRIPT
2 Correspondence: Ziv Gan-OrMontreal Neurological Institute, McGill University 1033 Pine Avenue, West,
Ludmer Pavilion, room 327 Montreal, QC, H3A 1A1 Phone: +1-514-398-6821
M
AN
US
CR
IP
T
AC
CE
PT
ED
ACCEPTED MANUSCRIPT
3Abstract: The current study aimed to examine whether the APOE ε4 allele, associated with dementia with Lewy bodies (DLB), and possibly with dementia in Parkinson’s disease (PD), is also associated with idiopathic REM sleep behavior disorder (RBD). Two SNPs, rs429358 and rs7412, were genotyped
in RBD patients (n=480) and in controls (n=823). APOE ε4 allele frequency was 0.14 among RBD
patients and 0.13 among controls (OR=1.11, 95% CI 0.88-1.40, p=0.41). APOE ε4 allele frequencies
were similar in those who converted to DLB (0.14) and those who converted to PD (0.12) or multiple
system atrophy (0.14, p=1.0). The APOE ε4 allele is neither a risk factor for RBD nor it is associated
with conversion from RBD to DLB or other synucleinopathies.
1. Introduction: Rapid eye movement (REM) sleep behavior disorder (RBD) is currently the strongest
clinical prodromal feature preceding the development of an overt synucleinopathy, including Parkinson’s disease (PD), dementia with Lewy bodies (DLB) or multiple system atrophy (MSA) (Iranzo, et al., 2014). One of the strongest genetic factors associated with DLB is the APOE epsilon4
(ε4) allele(Pickering-Brown, et al., 1994), and PD patients who carry this allele may be at increased risk
for developing dementia. Since both RBD and the APOE ε4 allele are possibly associated with DLB,
and with dementia in PD patients, we aimed to examine whether the APOE ε4 allele is associated with
RBD and conversion to DLB. See Supplementary file for detailed introduction and full list of references.
2. Methods: The study population included idiopathic RBD patients (n=480) and controls (n=823) of
European ancestry. RBD patients were diagnosed using clinical interview and polysomnography according to the ICSD-2 (International Classification of Sleep Disorders, version 2) criteria. The control group was composed of 253 elderly controls (age 59.5±9.8 years, matched to the available age at onset (AAO) of RBD, n=307, age 59.2±11.5), 510 young controls (age 34.0±6.5 years), and additional 60 controls with no available data on age. All control groups had nearly identical frequencies of the APOE
M
AN
US
CR
IP
T
AC
CE
PT
ED
ACCEPTED MANUSCRIPT
4individuals signed informed consent forms at enrollment, and the study protocols were approved by the respective institutional review boards. DNA was extracted using a standard salting-out protocol. Two single nucleotide polymorphisms (SNPs), rs429358 and rs7412, were genotyped using TaqMan SNP genotyping assays. Genotypes were called using the QuantStudio™ 7 Flex Real-Time PCR System and Software (v 1.0). Goodness of fit test with one degree of freedom was applied to look for deviation from the Hardy-Weinberg equilibrium (HWE) among the controls. Differences in APOE allele or carriage frequencies were analyzed using the Fisher’s exact test, and differences in continuous variables were analyzed using t-test. A logistic regression model with age and sex as covariates was also applied. All statistical analysis was done using SPSS statistics V.23 (IBM Inc.). Detailed methods can be found in the supplementary file.
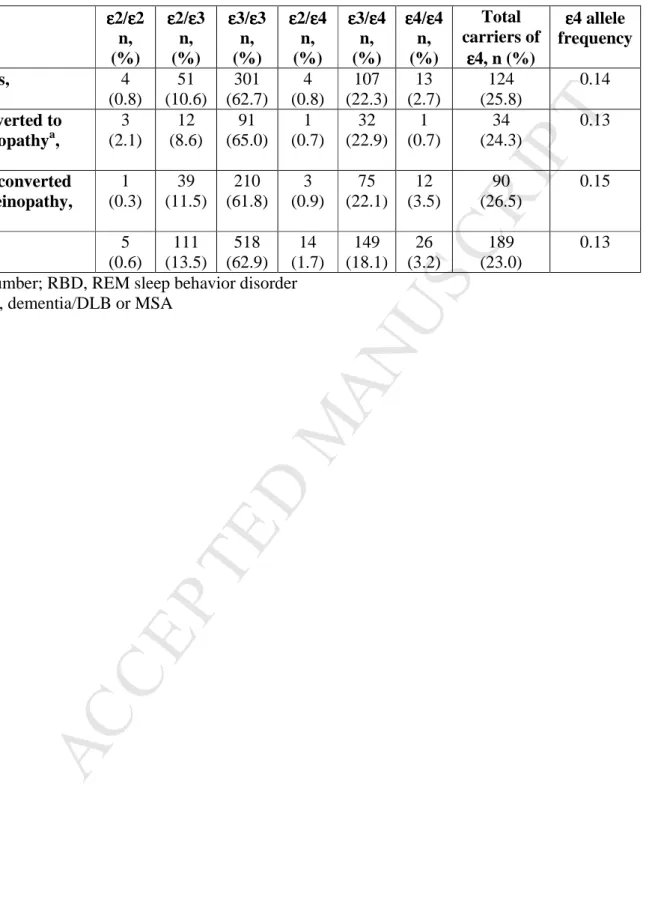
3. Results: The allele frequency of APOE ε4 was 0.14 among RBD patients and 0.13 among controls
(OR=1.11, 95% CI 0.88-1.40, p=0.41). Overall, 25.8% of RBD patients carried at least one APOE ε4
compared to 23.0% among controls (p=0.25, Fisher’s exact test), and there were more homozygous
carriers of the APOE ε4 allele among controls (3.2%) as compared to RBD patients (2.7%). Logistic
regression model adjusted for age and sex also demonstrated lack of association between APOE ε4
allele carriage and risk for RBD (OR = 1.25, 95% CI 0.87-1.79, p=0.23). There was no difference in
AAO when comparing carriers (n=88) and non-carriers (n=219) of the APOE ε4 allele (59.1 ± 8.4 vs.
59.3 ± 12.6 years, respectively, p=0.92, t-test). A total of 140 RBD patients (29.2%) were reported to have converted to either PD (n=98, 70% of the converters), dementia/DLB (n=28, 20%) or MSA (n=14,
10%). The carrier frequencies of one or more APOE ε4 in these groups were similar; 23.5%, 25.0% and
28.6%, respectively (p=0.91), and the allele frequencies were 0.12, 0.14 and 0.14 (p=1.0). The APOE ε4
allele frequency among those that did not convert was slightly higher, 0.15 (Table 1), with a total of
26.5% carriers of at least one APOE ε4 allele, compared to 24.3% among those who converted (p=0.65).
M
AN
US
CR
IP
T
AC
CE
PT
ED
ACCEPTED MANUSCRIPT
54. Discussion: Although RBD is a strong risk factor for developing DLB, and although DLB was
reported to be associated with the APOE ε4 allele, our results demonstrate lack of association between
the APOE ε4 allele and RBD or its age at onset. These and previous results further suggest that RBD
may have a distinct genetic background; it is associated with GBA mutations(Gan-Or, et al., 2015b), but
unlike PD it is not associated with LRRK2 mutations(Fernandez-Santiago, et al., 2016), and unlike DLB
it is not associated with the APOE ε4 allele. Thus far, GBA, SCARB2, and potentially SNCA (Gan-Or, et
al., 2015a) overlap between RBD, PD and DLB (Supplementary Figure 1, see Supplementary file). Whether RBD has additional, unique genetic factors that were not identified in PD or DLB cohorts is
still to be determined. Our current study identified similar frequencies of APOE ε4 allele in those who
progressed to PD, DLB and MSA, suggesting that APOE alleles do not affect the type of subsequent synucleinopathy. Our study has some limitations, and a more detailed discussion including full list of references can be found in the supplementary file. Our results support a distinct genetic background for RBD-associated neurodegeneration, probably suggesting a specific genetic association with synucleinopathy rather than tauopathy/amyloidopathy.
References:
Fernandez-Santiago, R., Iranzo, A., Gaig, C., Serradell, M., Fernandez, M., Tolosa, E., Santamaria, J., Ezquerra, M. 2016. Absence of LRRK2 mutations in a cohort of patients with idiopathic REM sleep behavior disorder. Neurology 86(11), 1072-3. doi:10.1212/WNL.0000000000002304.
Gan-Or, Z., Girard, S.L., Noreau, A., Leblond, C.S., Gagnon, J.F., Arnulf, I., Mirarchi, C., Dauvilliers, Y., Desautels, A., Mitterling, T., Cochen De Cock, V., Frauscher, B., Monaca, C., Hogl, B., Dion, P.A., Postuma, R.B., Montplaisir, J.Y., Rouleau, G.A. 2015a. Parkinson's Disease Genetic Loci in Rapid Eye Movement Sleep Behavior Disorder. J Mol Neurosci 56(3), 617-22. doi:10.1007/s12031-015-0569-7.
Gan-Or, Z., Mirelman, A., Postuma, R.B., Arnulf, I., Bar-Shira, A., Dauvilliers, Y., Desautels, A., Gagnon, J.F., Leblond, C.S., Frauscher, B., Alcalay, R.N., Saunders-Pullman, R., Bressman, S.B., Marder, K., Monaca, C., Hogl, B., Orr-Urtreger, A., Dion, P.A., Montplaisir, J.Y., Giladi, N., Rouleau, G.A. 2015b. GBA mutations are associated with Rapid Eye Movement Sleep Behavior Disorder. Ann Clin Transl Neurol 2(9), 941-5. doi:10.1002/acn3.228.
Iranzo, A., Fernandez-Arcos, A., Tolosa, E., Serradell, M., Molinuevo, J.L., Valldeoriola, F., Gelpi, E., Vilaseca, I., Sanchez-Valle, R., Llado, A., Gaig, C., Santamaria, J. 2014. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One 9(2), e89741.
doi:10.1371/journal.pone.0089741.
Pickering-Brown, S.M., Mann, D.M., Bourke, J.P., Roberts, D.A., Balderson, D., Burns, A., Byrne, J., Owen, F. 1994. Apolipoprotein E4 and Alzheimer's disease pathology in Lewy body disease and in other beta-amyloid-forming diseases. Lancet 343(8906), 1155.
M
AN
US
CR
IP
T
AC
CE
PT
ED
ACCEPTED MANUSCRIPT
6 Disclosure statementZGO received consultation fees from Sanofi/Genzyme. JYM reports grants from Merck, GlaxoSmithKline, received speaking honoraria from Valeant Pharmaceutical, and Otsuka Pharmaceutical, serves on the advisory boards of Sanofi-Aventis, Servier, Merck, Jazz Pharma, Valeant Pharma, Impax Laboratories, Glaxo-SmithKline, UCB Canada, received consultancy fees from Otsuka Pharma, and Valeant Pharma. JPR reports no conflict of interests. JP reports no conflict of interests. SCW received honoraria from Pfizer, Bristol-Myers Squibb, SmithKline Beecham and Eli Lilly. IA received speaker honoraria form UCB Pharma. SS reports no conflict of interests. YD is on the advisory board and received travel and consultancy fees from UCB Phrma, bioprojet, and Jazz Pharma. CSL reports no conflict of interests. MTH reports no conflict of interests. BH received grant from UCB, speaker honoraria from UCB, Otsuka, Abbvie, Lundbeck, Lilly, Mundipharma. Serving on advisory boards or consulting for Mundipharma, Axovant. Received travel support from Habel Medizintechnik, Vivisol. AS reports no conflict of interests. CCM received fees for serving on advisory board of UCB pharma, lecture fees from UCB Pharma, Orkyn. VCD received funding from Orkyn, LVL medical, Teva and UCB. MB reports no conflict of interests. LFS reports no conflict of interests. GP served on the advisory board of UCB pharma, Jazz pharmaceuticals and Bioproject. EA reports no conflict of interests. PY received honoraria for speakers bureaus by Sanofi Genzyme, Biomarin, UCB pharma, Medice, ResMed and Heinen und Loewenstein. Member of advisory boards for Sanofi Genzyme, Biomarin, Vanda and Medice. AH received travel support Habel Medizintechnik, received lecture honoraria from UCB, Heinen und Löwenstein. TRB reports no conflict of interests. SGE reports no conflict of interests. MR reports no conflict of interests. PAD reports no conflict of interests. AD received research grants from Novartis pharma, Jazz Pharmaceuticals, Biron soins du sommeil. Received speaker honoraria from UCB and Paladin labs.
Acknowledgements
We would like to thank all the participants in the study. This work was funded by a grant to ZGO from the Michael J. Fox Foundation for Parkinson’s research. Part of this work was funded by an interface grant to IA from INSERM. The French DNA collection was promoted by the Association pour le Développement et l'Organisation de la Recherche en Pneumologie et sur le Sommeil (ADOREPS), project PARAGEN, PI Isabelle Arnulf. The Oxford Discovery cohort was funded by the Monument Trust Discovery Award from Parkinson’s UK and supported by the National Institute for Health Research (NIHR), Oxford Biomedical Research Centre based at Oxford University Hospitals, NHS Trust, University of Oxford, and the Dementias and Neurodegenerative Diseases Research Network (DeNDRoN). Part of this work was funded by the Weston Brain Institute (grants to JYM and JP) and the J.L. Levesque Foundation (grant to JP). Part of this work was funded by grants to PY from the Lowensteinstiftung and the German Ministry of Education and Science (BMBF). ZGO is supported by a postdoctoral fellowship from the CIHR. JFG holds a Canada Research Chair on Cognitive Decline in Pathological Aging. GAR holds a Canada Research Chair in Genetics of the Nervous System and the Wilder Penfield Chair in Neurosciences. We thank Cynthia Bourassa, Sandra Laurent, Helene Catoire, Pascale Hince and Vessela Zaharieva for their assistance.
M
AN
US
CR
IP
T
AC
CE
PT
ED
ACCEPTED MANUSCRIPT
Table 1. APOE haplotypes in individuals with RBD and controls
APOE εεεε2/εεεε2 n, (%) εεεε2/εεεε3 n, (%) εεεε3/εεεε3 n, (%) εεεε2/εεεε4 n, (%) εεεε3/εεεε4 n, (%) εεεε4/εεεε4 n, (%) Total carriers of εεεε4, n (%) εεεε4 allele frequency RBD patients, n=480 4 (0.8) 51 (10.6) 301 (62.7) 4 (0.8) 107 (22.3) 13 (2.7) 124 (25.8) 0.14 RBD converted to synucleinopathya, n=140 3 (2.1) 12 (8.6) 91 (65.0) 1 (0.7) 32 (22.9) 1 (0.7) 34 (24.3) 0.13 RBD not converted to synucleinopathy, n=340 1 (0.3) 39 (11.5) 210 (61.8) 3 (0.9) 75 (22.1) 12 (3.5) 90 (26.5) 0.15 Controls, n=823 5 (0.6) 111 (13.5) 518 (62.9) 14 (1.7) 149 (18.1) 26 (3.2) 189 (23.0) 0.13 n, number; RBD, REM sleep behavior disorder
a
1
The dementia-associated APOE 4 allele is not associated with REM sleep behavior disorder
Ziv Gan-Or, MD, PhD,a,b,c, Jacques Y. Montplaisir, MD, PhD,d,e, Jay P. Ross, BSc,b Judes Poirier, PhD f,g, Simon C. Warby, PhD,4,5, Isabelle Arnulf, MD, PhD,h, Stephanie Strong, BSc,a, Yves Dauvilliers, MD, PhD,i, Claire S. Leblond, PhD,1,b, Michele T.M. Hu,MBBS, FRCP, PhD,j,k, Birgit Högl, MD,l, Ambra Stefani, MD,l, Christelle Charley Monaca, MD, PhD,m, Valérie Cochen De Cock, MD, PhD,n,o, Michel Boivin, PhD,p,q,Luigi Ferini-Strambi, MD, PhD,r,
Giuseppe Plazzi, MD, PhD,s,t, Elena Antelmi, MD,s, Peter Young, MD,u, Anna Heidbreder, MD,u, Thomas R Barber MA, MBBS, MRCP,j,k, Samuel G. Evetts BSc Hons, MSc,j,k, Michal Rolinski, BM BCh, BA Hons, MRCP,j,k, Patrick A. Dion, PhD,a,c, Alex Desautels, MD, PhD,d,v, Jean-François Gagnon, PhD,d,w, Nicolas Dupré, MD, MSc,x, Ronald B. Postuma, MD, MSc,c,y, and Guy A. Rouleau, MD, PhD,a,b,c.
Affiliations :
aMontreal Neurological Institute, McGill University, Montréal, QC, H3A 0G4, Canada, bDepartment of Human Genetics, McGill University, H3A 0G4, Montréal, QC, Canada, cDepartment of Neurology and neurosurgery, McGill University, Montréal, QC, H3A 0G4,
Canada, dCentre d’Études Avancées en Médecine du Sommeil, Hôpital du Sacré-Cœur de Montréal, Montréal, QC,H4J 1C5, Canada, eDepartment of Psychiatry, Université de Montréal, Montréal, QC, H3T 1J4, Canada, fDepartment of Psychiatry, McGill University, Montréal, QC,
H3A 0G4, Canada, gDouglas Mental Health University Institute, Montréal, QC, H4H 1R3, Canada,
hSleep Disorders Unit, Pitié Salpêtrière Hospital, Centre de Recherche de l’Institut du Cerveau et
de la Moelle Epinière and Sorbonne Universities, UPMC Paris 6 univ, Paris,75013, France, iSleep
Unit, National Reference Network for Narcolepsy, Department of Neurology Hôpital-Gui-de Chauliac, CHU Montpellier, INSERM U1061, Montpellier, 34000, France, jOxford Parkinson’s Disease Centre (OPDC), University of Oxford, Oxford, OX1 2JD, United Kingdom, kNuffield
Department of Clinical Neurosciences, University of Oxford, Oxford, OX1 2JD, United Kingdom,
lSleep Disorders Clinic, Department of Neurology, Medical University of Innsbruck, Innsbruck,
6020, Austria, mUniversity Lille north of France, Department of clinical neurophysiology and sleep center, CHU Lille, Lille, 59000, France, nSleep and neurology unit, Beau Soleil Clinic, Montpellier, 34070, France, oEuroMov, University of Montpellier, Montpellier, 34095, France,
pGRIP, École de psychologie, Université Laval, Québec city, QC, G1V 0A6, Canada, qInstitute of
Genetic, Neurobiological and Social Foundations of Child Development, Tomsk State University, Tomsk, 634050, Russia, rDepartment of Neurological Sciences, Università Vita-Salute San Raffaele, Milan, 20132, Italy, sDepartment of Biomedical and Neuromotor Sciences (DIBINEM),
Alma Mater Studiorum, University of Bologna, Bologna, 40126, Italy, tIRCCS, Institute of Neurological Sciences of Bologna, Bologna, 40139, Italy, uDepartment of Sleep Medicine and Neuromuscular Disorders, University of Muenster, 48149, Germany, vDepartment of
Neurosciences, Université de Montréal, Montréal, H3T 1J4, Canada, wDépartement de psychologie, Université du Québec à Montréal, Montréal, QC, H2L 2C4, Canada, xFaculté de
2
Médecine, Université Laval, CHU de Québec (Enfant-Jésus), Québec, QC, G1J 1Z4, Canada,
yDepartment of Neurology, Montreal General Hospital, Montréal, QC, H3G 1A4, Canada.
Correspondence:
Ziv Gan-Or
Montreal Neurological Institute, McGill University 1033 Pine Avenue, West,
Ludmer Pavilion, room 327 Montreal, QC, H3A 1A1 Phone: +1-514-398-6821
3
Abstract
A significant proportion of individuals with REM sleep behavior disorder (RBD) will progress to
dementia with Lewy bodies (DLB) and Parkinson’s disease (PD). We aimed to examine whether
the APOE 4 allele, associated with DLB, and possibly with dementia in PD, is also associated
with idiopathic RBD. The two SNPs tagging the different APOE alleles (rs429358 and rs7412)
were genotyped in individuals who were initially diagnosed with RBD (n=480) and in controls
(n=823). APOE 4 allele frequency was 0.14 among RBD patients and 0.13 among controls
(OR=1.11, 95% CI 0.88-1.40, p=0.41), and this lack of association remained after adjustment for
age and sex. Furthermore, allele frequencies of APOE 4 were similar in those who converted to
DLB (0.14) and those who converted to PD (0.12) or multiple system atrophy (0.14, p=1.0). The
APOE 4 allele is neither a risk factor for RBD nor it is associated with conversion from RBD to
DLB or other synucleinopathies.
4
Introduction
Rapid eye movement (REM) sleep behavior disorder (RBD), characterized by lack of muscle
atonia and enacting of dreams during REM sleep, is currently the strongest clinical prodromal
feature preceding the development of an overt synucleinopathy. With long term follow-up, more
than 80% of individuals with idiopathic RBD developed either Parkinson’s disease (PD), dementia
with Lewy bodies (DLB) or multiple system atrophy (MSA) (Iranzo, et al., 2014,Schenck, et al.,
2013). It was suggested that RBD may define a subtype of PD patients (Fereshtehnejad, et al.,
2015,Gagnon, et al., 2004) with cognitive decline (Gagnon, et al., 2009,Vendette, et al., 2007),
dementia (Anang, et al., 2014), hallucinations (Sixel-Doring, et al., 2011) and autonomic
dysfunction (Postuma, et al., 2008), as compared to PD patients without RBD. In addition,
pathological studies in brains of PD patients with and without RBD demonstrated a more
widespread -synuclein accumulation in those associated with RBD (Postuma, et al., 2015a).
If indeed RBD represents a subtype of PD, or a subtype of synucleinopathy, it is possible
that it has specific genetic background. A preliminary study that examined the association of RBD
with several genetic risk factors for PD identified an association mainly with MAPT and SCARB2,
and marginal or lack of association with other markers (Gan-Or, et al., 2015a). A recent study
suggested that RBD is associated with mutations in GBA in both idiopathic RBD and PD cohorts
(Gan-Or, et al., 2015b). This association was stronger than the association of GBA mutations with
PD in a similar population (Noreau, et al., 2011), suggesting that GBA may be one of the genetic
factors that is more specific to RBD. Furthermore, the association of GBA mutations with DLB
(Nalls, et al., 2013) also seems to be stronger than the association with PD (Sidransky, et al., 2009).
5
2016,Pont-Sunyer, et al., 2015,Saunders-Pullman, et al., 2015), further supporting the hypothesis
that RBD has a distinct genetic background.
One of the strongest genetic factors associated with DLB is the APOE epsilon4 (allele
(Pickering-Brown, et al., 1994), and PD patients who carry this allele may be at increased risk for
developing dementia (Pankratz, et al., 2006), although negative results were also reported for
these associations (Jasinska-Myga, et al., 2007,Lovati, et al., 2010). Since both RBD and the APOE
allele are possibly associated with DLB, and with dementia in PD patients, we aimed to examine whether the APOE 4 allele is associated with RBD and conversion to DLB.
Methods
Population
The study population included consecutively recruited, unrelated idiopathic RBD patients (n=480)
and controls (n=823) of European ancestry. RBD patients were collected by collaborators from the
international RBD study group and were diagnosed using clinical interview and polysomnography
according to the ICSD-2 (International Classification of Sleep Disorders, version 2)
criteria.(Thorpy, 2012) The control group was composed of 253 elderly controls (age 59.5±9.8
years, matched to the available age at onset (AAO) of RBD, n=307, age 59.2±11.5), 510 young
controls (age 34.0±6.5 years), and additional 60 controls with no available data on age. However,
all control groups had nearly identical frequencies of the APOE 4 allele (0.13, 0.13 and 0.14,
respectively), suggesting lack of age-effect, which allowed us to analyze all controls combined.
All individuals signed informed consent forms at enrollment, and the study protocols were
6
Genotyping
DNA was extracted using a standard salting-out protocol. To determine the APOE haplotypes, two
tagging single nucleotide polymorphisms (SNPs), rs429358 and rs7412, were genotyped using
TaqMan SNP genotyping assays (C___3084793_20 and C____904973_10, respectively,
ThermoFisher Scientific Inc.) according to the manufacturer's instructions. Genotypes were called
using the QuantStudio™ 7 Flex Real-Time PCR System and Software (v 1.0). Carriers of T in rs429358 and T in rs7412 were determined as carriers of the 2 allele, carriers of T in rs429358
and C in rs7412 were determined as carriers of the 3 allele, and carriers of C in rs429358 and C
in rs7412 were determined as carriers of the 4 allele. Of this cohort, the GBA gene was sequenced
in 265 RBD patients (Gan-Or, et al., 2015b), and nine PD-associated SNPs were genotyped in 261
patients(Gan-Or, et al., 2015a).
Statistical analysis
Categorical variables are presented as percentage or frequencies, whereas continuous variables are
presented as mean ± standard deviation. Goodness of fit test with one degree of freedom was
applied to look for deviation from the Hardy-Weinberg equilibrium (HWE) among the controls.
Differences in APOE allele or carriage frequencies were analyzed using the Fisher’s exact test,
and differences in continuous variables were analyzed using t-test. To further avoid a potential
bias due to age, and since sex distribution was different among patients with RBD vs. controls, a
logistic regression model with age and sex as covariates was also applied. All statistical analysis
was done using SPSS statistics V.23 (IBM Inc.).
7
Lack of association between the APOE 4, RBD risk and its age at onset
Table 1 details the different APOE alleles in RBD patients and controls. The frequency of the two
SNPs defining the APOE alleles did not deviate from HWE. The allele frequency of APOE 4 was
0.14 among RBD patients and 0.13 among controls (OR=1.11, 95% CI 0.88-1.40, p=0.41).
Overall, 25.8% of RBD patients carried at least one APOE 4 compared to 23.0% among controls
(p=0.25, Fisher’s exact test), and there were more homozygous carriers of the APOE 4 allele
among controls (3.2%) as compared to RBD patients (2.7%). Logistic regression model adjusted
for age and sex also demonstrated lack of association between APOE 4 allele carriage and risk
for RBD (OR = 1.25, 95% CI 0.87-1.79, p=0.23). Data on age at onset (AAO) of RBD was
available for 307 individuals, and there was no difference in AAO when comparing carriers (n=88)
and non-carriers (n=219) of the APOE 4 allele (59.1 ± 8.4 vs. 59.3 ± 12.6 years, respectively,
p=0.92, t-test).
The APOE 4 allele is not associated with conversion to PD, DLB or MSA.
Since most of the patients in our cohort are being followed-up longitudinally, we examined
whether the APOE 4 allele is associated with conversion to either PD, dementia/DLB, or MSA.
A total of 140 RBD patients (29.2%) were reported to have converted to either PD (n=98, 70% of
the converters), dementia/DLB (n=28, 20%) or MSA (n=14, 10%). The carrier frequencies of one
or more APOE 4 in these groups were similar; 23.5%, 25.0% and 28.6%, respectively (p=0.91),
and the allele frequencies were 0.12, 0.14 and 0.14 (p=1.0). The APOE 4 allele frequency among
those that did not convert was slightly higher, 0.15 (Table 1), with a total of 26.5% carriers of at
8
Discussion
Although RBD is a strong risk factor for developing DLB (Iranzo, et al., 2014,Postuma, et al.,
2015b). and although DLB was reported to be associated with the APOE 4 allele (Kobayashi, et
al., 2011,Lane, et al., 2009,Pickering-Brown, et al., 1994), our results demonstrate lack of
association between the APOE 4 allele and RBD or its age at onset. These and previous results
(Fernandez-Santiago, et al., 2016,Gan-Or, et al., 2015a,Gan-Or, et al., 2015b,Saunders-Pullman,
et al., 2015) further suggest that RBD may have a distinct genetic background; it is associated with
GBA mutations (Gan-Or, et al., 2015b), but unlike PD it is not associated with LRRK2 mutations
(Fernandez-Santiago, et al., 2016,Saunders-Pullman, et al., 2015), and unlike DLB it is not
associated with the APOE 4 allele. Thus far, GBA, SCARB2, and potentially SNCA overlap
between RBD, PD and DLB (Figure 1) (Bras, et al., 2014,Gan-Or, et al., 2015a,Gan-Or, et al.,
2015b). Whether RBD has additional, unique genetic factors that were not identified in PD or DLB
cohorts is still to be determined.
Since PD patients with RBD are likely to develop dementia and hallucinations (Anang, et
al., 2014,Sinforiani, et al., 2008), eventually presenting a phenotype similar to DLB, and based on
the current and previous genetic and post-mortem results (Postuma, et al., 2015a), we hypothesize
that RBD-associated synucleinopathy (the central common area in Figure 1) is the same
clinical-pathological entity, whether it is defined as parkinsonism first with subsequent dementia and
hallucinations, or whether it is defined as DLB with subsequent parkinsonism. In that sense, RBD
can be considered as a marker for diffuse synucleinopathy, which may be a better description of
9
also demonstrated a more diffuse synucleinopathy (Choi, et al., 2011,Neumann, et al., 2009,Wong,
et al., 2004), further supporting this notion. However, others suggest that PD and DLB should
remain separate entities, at least until better genetic, molecular and clinical data will allow better
definitions of these diseases and their potential subgroups (Boeve, et al., 2016). It is possible that
stochastic events, or other genetic or environmental factors, determine whether -synuclein will
first be deposited in brain areas associated with dementia and later in the areas associated with
parkinsonism, or vice versa (Lai, et al., 2008,Lai and Siegel, 2003). The observation that
-synuclein can spread in the brain in a prion-like fashion (Bernis, et al., 2015,Danzer, et al.,
2009,Freundt, et al., 2012) may support a stochastic progression hypothesis, yet additional
neuro-pathological studies are needed to examine this possibility. Therefore, there are two possible
explanations for the lack of association between the APOE 4 allele and conversion to DLB in our
cohort. First, as previously suggested (Bras, et al., 2014), it is possible that the association of the
APOE 4 allele with DLB is due to the component of DLB patients who also have a tauopathy,
and that the association of RBD with DLB is with those who have more pure synucleinopathy.
Alternatively, since the majority of our cohort had not yet converted to an overt synucleinopathy,
it is possible that once a larger number would convert, an association between APOE 4 allele and
conversion to DLB may arise. Hence, a follow-up study will be needed to determine this
possibility.
The association of RBD with the more devastating synucleinopathy, MSA, also
necessitates more study. Whether unique genetic or environmental factors affect the risk to
progress from RBD to MSA is still unknown. Our current study identified similar frequencies of
APOE 4 allele in those who progressed to PD, DLB and MSA, suggesting that APOE is not one
10
MSA as well,(Mitsui, et al., 2015) however this observation is awaiting replications in additional
cohorts.
Our study has some limitations. The control population was younger than the RBD group.
To tackle this limitation, we took two approaches. First, we demonstrated that the frequencies of
the APOE 4 allele were similar across generations (i.e. in the elderly and young control groups),
which rules out a potential bias. Furthermore, we also performed a logistic regression model with
adjustment for age, which further demonstrated lack of association between the APOE 4 allele
and RBD. Another possible limitation would stem from RBD patients being recruited in multiple
centers, which could have led to a potential population dependent bias if some cohorts are enriched
in APOE 4 allele carriers. However, since the frequencies of the APOE 4 allele were similar
across centers, this possibility was ruled out.
To conclude, our results support a distinct genetic background for RBD-associated
neurodegeneration, probably suggesting a specific genetic association with synucleinopathy rather
than tauopathy/amyloidopathy. To examine the hypotheses raised by the current and previous
work, larger studies are necessary, including genome wide association and next-generation
11
Acknowledgements
We would like to thank all the participants in the study. This work was funded by a grant to ZGO from the Michael J. Fox Foundation for Parkinson’s research. Part of this work was funded by an interface grant to IA from INSERM. The French DNA collection was promoted by the Association pour le Développement et l'Organisation de la Recherche en Pneumologie et sur le Sommeil (ADOREPS), project PARAGEN, PI Isabelle Arnulf. The Oxford Discovery cohort was funded by the Monument Trust Discovery Award from Parkinson’s UK and supported by the National Institute for Health Research (NIHR), Oxford Biomedical Research Centre based at Oxford University Hospitals, NHS Trust, University of Oxford, and the Dementias and Neurodegenerative Diseases Research Network (DeNDRoN). Part of this work was funded by the Weston Brain Institute (grants to JYM and JP) and the J.L. Levesque Foundation (grant to JP). Part of this work was funded by grants to PY from the Lowensteinstiftung and the German Ministry of Education and Science (BMBF). ZGO is supported by a postdoctoral fellowship from the CIHR. JFG holds a Canada Research Chair on Cognitive Decline in Pathological Aging. GAR holds a Canada Research Chair in Genetics of the Nervous System and the Wilder Penfield Chair in Neurosciences. We thank Cynthia Bourassa, Sandra Laurent, Helene Catoire, Pascale Hince and Vessela Zaharieva for their assistance.
Financial Disclosures
ZGO received consultation fees from Sanofi/Genzyme. JYM reports grants from Merck, GlaxoSmithKline, received speaking honoraria from Valeant Pharmaceutical, and Otsuka Pharmaceutical, serves on the advisory boards of Sanofi-Aventis, Servier, Merck, Jazz Pharma, Valeant Pharma, Impax Laboratories, Glaxo-SmithKline, UCB Canada, received consultancy fees from Otsuka Pharma, and Valeant Pharma. JPR reports no conflict of interests. JP reports no conflict of interests. SCW received honoraria from Pfizer, Bristol-Myers Squibb, SmithKline Beecham and Eli Lilly. IA received speaker honoraria form UCB Pharma. SS reports no conflict of interests. YD is on the advisory board and received travel and consultancy fees from UCB Phrma, bioprojet, and Jazz Pharma. CSL reports no conflict of interests. MTH reports no conflict of interests. BH received grant from UCB, speaker honoraria from UCB, Otsuka, Abbvie, Lundbeck, Lilly, Mundipharma. Serving on advisory boards or consulting for Mundipharma, Axovant. Received travel support from Habel Medizintechnik, Vivisol. AS reports no conflict of interests. CCM received fees for serving on advisory board of UCB pharma, lecture fees from UCB Pharma, Orkyn. VCD received funding from Orkyn, LVL medical, Teva and UCB. MB reports no conflict of interests. LFS reports no conflict of interests. GP served on the advisory board of UCB pharma, Jazz pharmaceuticals and Bioproject. EA reports no conflict of interests. PY received honoraria for speakers bureaus by Sanofi Genzyme, Biomarin, UCB pharma, Medice, ResMed and Heinen und Loewenstein. Member of advisory boards for Sanofi Genzyme, Biomarin, Vanda and Medice. AH received travel support Habel Medizintechnik, received lecture honoraria from UCB, Heinen und Löwenstein. TRB reports no conflict of interests. SGE reports no conflict of interests. MR reports no conflict of interests. PAD reports no conflict of interests. AD received
12
research grants from Novartis pharma, Jazz Pharmaceuticals, Biron soins du sommeil. Received speaker honoraria from UCB and Paladin labs.
13
References
Anang, J.B., Gagnon, J.F., Bertrand, J.A., Romenets, S.R., Latreille, V., Panisset, M., Montplaisir, J., Postuma, R.B. 2014. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 83(14), 1253-60. doi:10.1212/WNL.0000000000000842.
Bernis, M.E., Babila, J.T., Breid, S., Wusten, K.A., Wullner, U., Tamguney, G. 2015. Prion-like propagation of human brain-derived synuclein in transgenic mice expressing human wild-type alpha-synuclein. Acta Neuropathol Commun 3, 75. doi:10.1186/s40478-015-0254-7.
Boeve, B.F., Dickson, D.W., Duda, J.E., Ferman, T.J., Galasko, D.R., Galvin, J.E., Goldman, J.G., Growdon, J.H., Hurtig, H.I., Kaufer, D.I., Kantarci, K., Leverenz, J.B., Lippa, C.F., Lopez, O.L., McKeith, I.G., Singleton, A.B., Taylor, A., Tsuang, D., Weintraub, D., Zabetian, C.P. 2016. Arguing against the proposed definition changes of PD. Mov Disord. doi:10.1002/mds.26721.
Bras, J., Guerreiro, R., Darwent, L., Parkkinen, L., Ansorge, O., Escott-Price, V., Hernandez, D.G., Nalls, M.A., Clark, L.N., Honig, L.S., Marder, K., Van Der Flier, W.M., Lemstra, A., Scheltens, P., Rogaeva, E., St George-Hyslop, P., Londos, E., Zetterberg, H., Ortega-Cubero, S., Pastor, P., Ferman, T.J., Graff-Radford, N.R., Ross, O.A., Barber, I., Braae, A., Brown, K., Morgan, K., Maetzler, W., Berg, D., Troakes, C., Al-Sarraj, S., Lashley, T., Compta, Y., Revesz, T., Lees, A., Cairns, N., Halliday, G.M., Mann, D., Pickering-Brown, S., Dickson, D.W., Singleton, A., Hardy, J. 2014. Genetic analysis implicates APOE, SNCA and suggests lysosomal dysfunction in the etiology of dementia with Lewy bodies. Hum Mol Genet 23(23), 6139-46.
doi:10.1093/hmg/ddu334.
Choi, J.H., Stubblefield, B., Cookson, M.R., Goldin, E., Velayati, A., Tayebi, N., Sidransky, E. 2011. Aggregation of alpha-synuclein in brain samples from subjects with glucocerebrosidase mutations. Mol Genet Metab 104(1-2), 185-8. doi:10.1016/j.ymgme.2011.06.008.
Danzer, K.M., Krebs, S.K., Wolff, M., Birk, G., Hengerer, B. 2009. Seeding induced by alpha-synuclein oligomers provides evidence for spreading of alpha-synuclein pathology. J Neurochem 111(1), 192-203. doi:10.1111/j.1471-4159.2009.06324.x.
Fereshtehnejad, S.M., Romenets, S.R., Anang, J.B., Latreille, V., Gagnon, J.F., Postuma, R.B. 2015. New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression: A Prospective Cohort Comparison With Other Phenotypes. JAMA Neurol 72(8), 863-73.
doi:10.1001/jamaneurol.2015.0703.
Fernandez-Santiago, R., Iranzo, A., Gaig, C., Serradell, M., Fernandez, M., Tolosa, E., Santamaria, J., Ezquerra, M. 2016. Absence of LRRK2 mutations in a cohort of patients with idiopathic REM sleep behavior disorder. Neurology 86(11), 1072-3. doi:10.1212/WNL.0000000000002304. Freundt, E.C., Maynard, N., Clancy, E.K., Roy, S., Bousset, L., Sourigues, Y., Covert, M., Melki, R.,
Kirkegaard, K., Brahic, M. 2012. Neuron-to-neuron transmission of alpha-synuclein fibrils through axonal transport. Ann Neurol 72(4), 517-24. doi:10.1002/ana.23747.
Gagnon, J.F., Fantini, M.L., Bedard, M.A., Petit, D., Carrier, J., Rompre, S., Decary, A., Panisset, M., Montplaisir, J. 2004. Association between waking EEG slowing and REM sleep behavior disorder in PD without dementia. Neurology 62(3), 401-6.
Gagnon, J.F., Vendette, M., Postuma, R.B., Desjardins, C., Massicotte-Marquez, J., Panisset, M.,
Montplaisir, J. 2009. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson's disease. Ann Neurol 66(1), 39-47. doi:10.1002/ana.21680.
Gan-Or, Z., Girard, S.L., Noreau, A., Leblond, C.S., Gagnon, J.F., Arnulf, I., Mirarchi, C., Dauvilliers, Y., Desautels, A., Mitterling, T., Cochen De Cock, V., Frauscher, B., Monaca, C., Hogl, B., Dion, P.A., Postuma, R.B., Montplaisir, J.Y., Rouleau, G.A. 2015a. Parkinson's Disease Genetic Loci in Rapid Eye Movement Sleep Behavior Disorder. J Mol Neurosci 56(3), 617-22. doi:10.1007/s12031-015-0569-7.
14
Gan-Or, Z., Mirelman, A., Postuma, R.B., Arnulf, I., Bar-Shira, A., Dauvilliers, Y., Desautels, A., Gagnon, J.F., Leblond, C.S., Frauscher, B., Alcalay, R.N., Saunders-Pullman, R., Bressman, S.B., Marder, K., Monaca, C., Hogl, B., Orr-Urtreger, A., Dion, P.A., Montplaisir, J.Y., Giladi, N., Rouleau, G.A. 2015b. GBA mutations are associated with Rapid Eye Movement Sleep Behavior Disorder. Ann Clin Transl Neurol 2(9), 941-5. doi:10.1002/acn3.228.
Iranzo, A., Fernandez-Arcos, A., Tolosa, E., Serradell, M., Molinuevo, J.L., Valldeoriola, F., Gelpi, E., Vilaseca, I., Sanchez-Valle, R., Llado, A., Gaig, C., Santamaria, J. 2014. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One 9(2), e89741. doi:10.1371/journal.pone.0089741.
Jasinska-Myga, B., Opala, G., Goetz, C.G., Tustanowski, J., Ochudlo, S., Gorzkowska, A., Tyrpa, J. 2007. Apolipoprotein E gene polymorphism, total plasma cholesterol level, and Parkinson disease dementia. Arch Neurol 64(2), 261-5. doi:10.1001/archneur.64.2.261.
Kobayashi, S., Tateno, M., Park, T.W., Utsumi, K., Sohma, H., Ito, Y.M., Kokai, Y., Saito, T. 2011.
Apolipoprotein E4 frequencies in a Japanese population with Alzheimer's disease and dementia with Lewy bodies. PLoS One 6(4), e18569. doi:10.1371/journal.pone.0018569.
Lai, Y.Y., Hsieh, K.C., Nguyen, D., Peever, J., Siegel, J.M. 2008. Neurotoxic lesions at the ventral
mesopontine junction change sleep time and muscle activity during sleep: an animal model of motor disorders in sleep. Neuroscience 154(2), 431-43. doi:10.1016/j.neuroscience.2008.03.085. Lai, Y.Y., Siegel, J.M. 2003. Physiological and anatomical link between Parkinson-like disease and REM
sleep behavior disorder. Mol Neurobiol 27(2), 137-52. doi:10.1385/MN:27:2:137.
Lane, R., He, Y., Morris, C., Leverenz, J.B., Emre, M., Ballard, C. 2009. BuChE-K and APOE epsilon4 allele frequencies in Lewy body dementias, and influence of genotype and hyperhomocysteinemia on cognitive decline. Mov Disord 24(3), 392-400. doi:10.1002/mds.22357.
Lovati, C., Galimberti, D., Albani, D., Bertora, P., Venturelli, E., Cislaghi, G., Guidi, I., Fenoglio, C., Cortini, F., Clerici, F., Finazzi, D., Forloni, G., Scarpini, E., Mariani, C. 2010. APOE epsilon2 and epsilon4 influence the susceptibility for Alzheimer's disease but not other dementias. Int J Mol Epidemiol Genet 1(3), 193-200.
Mitsui, J., Matsukawa, T., Sasaki, H., Yabe, I., Matsushima, M., Durr, A., Brice, A., Takashima, H., Kikuchi, A., Aoki, M., Ishiura, H., Yasuda, T., Date, H., Ahsan, B., Iwata, A., Goto, J., Ichikawa, Y.,
Nakahara, Y., Momose, Y., Takahashi, Y., Hara, K., Kakita, A., Yamada, M., Takahashi, H.,
Onodera, O., Nishizawa, M., Watanabe, H., Ito, M., Sobue, G., Ishikawa, K., Mizusawa, H., Kanai, K., Hattori, T., Kuwabara, S., Arai, K., Koyano, S., Kuroiwa, Y., Hasegawa, K., Yuasa, T., Yasui, K., Nakashima, K., Ito, H., Izumi, Y., Kaji, R., Kato, T., Kusunoki, S., Osaki, Y., Horiuchi, M., Kondo, T., Murayama, S., Hattori, N., Yamamoto, M., Murata, M., Satake, W., Toda, T., Filla, A.,
Klockgether, T., Wullner, U., Nicholson, G., Gilman, S., Tanner, C.M., Kukull, W.A., Stern, M.B., Lee, V.M., Trojanowski, J.Q., Masliah, E., Low, P.A., Sandroni, P., Ozelius, L.J., Foroud, T., Tsuji, S. 2015. Variants associated with Gaucher disease in multiple system atrophy. Ann Clin Transl Neurol 2(4), 417-26. doi:10.1002/acn3.185.
Nalls, M.A., Duran, R., Lopez, G., Kurzawa-Akanbi, M., McKeith, I.G., Chinnery, P.F., Morris, C.M., Theuns, J., Crosiers, D., Cras, P., Engelborghs, S., De Deyn, P.P., Van Broeckhoven, C., Mann, D.M., Snowden, J., Pickering-Brown, S., Halliwell, N., Davidson, Y., Gibbons, L., Harris, J., Sheerin, U.M., Bras, J., Hardy, J., Clark, L., Marder, K., Honig, L.S., Berg, D., Maetzler, W., Brockmann, K., Gasser, T., Novellino, F., Quattrone, A., Annesi, G., De Marco, E.V., Rogaeva, E., Masellis, M., Black, S.E., Bilbao, J.M., Foroud, T., Ghetti, B., Nichols, W.C., Pankratz, N., Halliday, G., Lesage, S., Klebe, S., Durr, A., Duyckaerts, C., Brice, A., Giasson, B.I., Trojanowski, J.Q., Hurtig, H.I., Tayebi, N., Landazabal, C., Knight, M.A., Keller, M., Singleton, A.B., Wolfsberg, T.G., Sidransky, E. 2013. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol 70(6), 727-35. doi:10.1001/jamaneurol.2013.1925.
15
Neumann, J., Bras, J., Deas, E., O'Sullivan, S.S., Parkkinen, L., Lachmann, R.H., Li, A., Holton, J., Guerreiro, R., Paudel, R., Segarane, B., Singleton, A., Lees, A., Hardy, J., Houlden, H., Revesz, T., Wood, N.W. 2009. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain 132(Pt 7), 1783-94. doi:10.1093/brain/awp044.
Noreau, A., Riviere, J.B., Diab, S., Dion, P.A., Panisset, M., Soland, V., Jodoin, N., Langlois, M., Chouinard, S., Dupre, N., Rouleau, G.A. 2011. Glucocerebrosidase mutations in a French-Canadian
Parkinson's disease cohort. Can J Neurol Sci 38(5), 772-3.
Pankratz, N., Byder, L., Halter, C., Rudolph, A., Shults, C.W., Conneally, P.M., Foroud, T., Nichols, W.C. 2006. Presence of an APOE4 allele results in significantly earlier onset of Parkinson's disease and a higher risk with dementia. Mov Disord 21(1), 45-9. doi:10.1002/mds.20663.
Pickering-Brown, S.M., Mann, D.M., Bourke, J.P., Roberts, D.A., Balderson, D., Burns, A., Byrne, J., Owen, F. 1994. Apolipoprotein E4 and Alzheimer's disease pathology in Lewy body disease and in other beta-amyloid-forming diseases. Lancet 343(8906), 1155.
Pont-Sunyer, C., Iranzo, A., Gaig, C., Fernandez-Arcos, A., Vilas, D., Valldeoriola, F., Compta, Y., Fernandez-Santiago, R., Fernandez, M., Bayes, A., Calopa, M., Casquero, P., de Fabregues, O., Jauma, S., Puente, V., Salamero, M., Jose Marti, M., Santamaria, J., Tolosa, E. 2015. Sleep Disorders in Parkinsonian and Nonparkinsonian LRRK2 Mutation Carriers. PLoS One 10(7), e0132368. doi:10.1371/journal.pone.0132368.
Postuma, R.B., Adler, C.H., Dugger, B.N., Hentz, J.G., Shill, H.A., Driver-Dunckley, E., Sabbagh, M.N., Jacobson, S.A., Belden, C.M., Sue, L.I., Serrano, G., Beach, T.G. 2015a. REM sleep behavior disorder and neuropathology in Parkinson's disease. Mov Disord 30(10), 1413-7.
doi:10.1002/mds.26347.
Postuma, R.B., Gagnon, J.F., Vendette, M., Charland, K., Montplaisir, J. 2008. Manifestations of Parkinson disease differ in association with REM sleep behavior disorder. Mov Disord 23(12), 1665-72. doi:10.1002/mds.22099.
Postuma, R.B., Iranzo, A., Hogl, B., Arnulf, I., Ferini-Strambi, L., Manni, R., Miyamoto, T., Oertel, W., Dauvilliers, Y., Ju, Y.E., Puligheddu, M., Sonka, K., Pelletier, A., Santamaria, J., Frauscher, B., Leu-Semenescu, S., Zucconi, M., Terzaghi, M., Miyamoto, M., Unger, M.M., Carlander, B., Fantini, M.L., Montplaisir, J.Y. 2015b. Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: a multicenter study. Ann Neurol 77(5), 830-9. doi:10.1002/ana.24385.
Saunders-Pullman, R., Alcalay, R.N., Mirelman, A., Wang, C., Luciano, M.S., Ortega, R.A., Glickman, A., Raymond, D., Mejia-Santana, H., Doan, N., Johannes, B., Yasinovsky, K., Ozelius, L., Clark, L., Orr-Utreger, A., Marder, K., Giladi, N., Bressman, S.B., Consortium, A.L. 2015. REM sleep behavior disorder, as assessed by questionnaire, in G2019S LRRK2 mutation PD and carriers. Mov Disord 30(13), 1834-9. doi:10.1002/mds.26413.
Schenck, C.H., Boeve, B.F., Mahowald, M.W. 2013. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med 14(8), 744-8. doi:10.1016/j.sleep.2012.10.009.
Sidransky, E., Nalls, M.A., Aasly, J.O., Aharon-Peretz, J., Annesi, G., Barbosa, E.R., Bar-Shira, A., Berg, D., Bras, J., Brice, A., Chen, C.M., Clark, L.N., Condroyer, C., De Marco, E.V., Durr, A., Eblan, M.J., Fahn, S., Farrer, M.J., Fung, H.C., Gan-Or, Z., Gasser, T., Gershoni-Baruch, R., Giladi, N., Griffith, A., Gurevich, T., Januario, C., Kropp, P., Lang, A.E., Lee-Chen, G.J., Lesage, S., Marder, K., Mata, I.F., Mirelman, A., Mitsui, J., Mizuta, I., Nicoletti, G., Oliveira, C., Ottman, R., Orr-Urtreger, A., Pereira, L.V., Quattrone, A., Rogaeva, E., Rolfs, A., Rosenbaum, H., Rozenberg, R., Samii, A., Samaddar, T., Schulte, C., Sharma, M., Singleton, A., Spitz, M., Tan, E.K., Tayebi, N., Toda, T., Troiano, A.R., Tsuji, S., Wittstock, M., Wolfsberg, T.G., Wu, Y.R., Zabetian, C.P., Zhao, Y., Ziegler,
16
S.G. 2009. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med 361(17), 1651-61. doi:10.1056/NEJMoa0901281.
Sinforiani, E., Pacchetti, C., Zangaglia, R., Pasotti, C., Manni, R., Nappi, G. 2008. REM behavior disorder, hallucinations and cognitive impairment in Parkinson's disease: a two-year follow up. Mov Disord 23(10), 1441-5. doi:10.1002/mds.22126.
Sixel-Doring, F., Trautmann, E., Mollenhauer, B., Trenkwalder, C. 2011. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology 77(11), 1048-54.
doi:10.1212/WNL.0b013e31822e560e.
Thorpy, M.J. 2012. Classification of sleep disorders. Neurotherapeutics 9(4), 687-701. doi:10.1007/s13311-012-0145-6.
Vendette, M., Gagnon, J.F., Decary, A., Massicotte-Marquez, J., Postuma, R.B., Doyon, J., Panisset, M., Montplaisir, J. 2007. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology 69(19), 1843-9.
doi:10.1212/01.wnl.0000278114.14096.74.
Wong, K., Sidransky, E., Verma, A., Mixon, T., Sandberg, G.D., Wakefield, L.K., Morrison, A., Lwin, A., Colegial, C., Allman, J.M., Schiffmann, R. 2004. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab 82(3), 192-207.
17
Figure 1. Venn diagram of the genetic overlap between PD, DLB and RBD.
While GBA mutations, SCARB2 and possibly SNCA variants are associated with all three
conditions, other genetic variants such as APOE 4 in DLB and LRRK2 mutations in PD are
distinctively associated with each condition but not with RBD. It is therefore likely that
RBD-associated neurodegeneration (the overlapping area of PD, DLB and RBD) may have distinct
genetic background. While thus far no genetic variants that are uniquely associated with RBD were
discovered, it is possible that such genetic risk factors do exist, and that they were not discovered