Phosphonium/Ammonium-Based Ionic Liquids for
Rare Earth Minerals Beneficiation: Case of Monazite
and Bastnäsite
Thèse
Dariush Azizi
Doctorat en génie chimique
Philosophiae doctor (Ph. D.)
Québec, Canada
Phosphonium/Ammonium Based Ionic Liquids for
Rare Erath Minerals Beneficiation: Case of Monazite
and Bastnäsite
Thèse
Dariush Azizi
Sous la direction de :
Résumé
Cette thèse de doctorat porte sur l'application des liquides ioniques à base de phosphonium et d'ammonium pour l'enrichissement des minéraux à base d'éléments de terres rares. Le manuscrit de thèse a été divisé en quatre parties principales. Tout d'abord, l'utilisation des liquides ioniques à base de phosphonium et d'ammonium a été explorée dans trois procédés différents pour la valorisation des minéraux de terres rares. Ces trois approches ont été examinées pour des minéraux à base d’éléments de terres rares contenus dans un minerai complexe en provenance du gisement Niobec au Québec ainsi que des minéraux modèles de monazite et de bastnäsite associés à la gangue du minerai Niobec. Par la suite, l'application de ces liquides ioniques à l'extraction par solvant des éléments des terres rares a été étudiée par simulation à l’aide d’outil de chimie quantique.
Dans le premier cas de valorisation des minéraux de terres rares, l'application d'un liquide ionique à base de phosphonium /ammonium comme collecteur aqueux pour la flottation par moussage de minéraux des terres rares a été étudiée. Dans cette étude, le liquide ionique a été identifié pour posséder des performances supérieures à celles offertes par les collecteurs classiques utilisés en industrie comme les collecteurs dérivés de l'acide hydroxamique industriel. Les interactions anioniques et cationiques du liquide ionique durant la flottation des minéraux d’éléments de terres rares ont été scrutées en détail pour révéler qu’une voie de synergie interne explique en partie les résultats de la flottation en terme de chimisorption de la partie anionique et de la physisorption et de la partie cationique du liquide ionique.
Dans le deuxième cas d'enrichissement des minéraux de terres rares, le potentiel de la séparation minérale liquide (aqueuse)-liquide (organique) au moyen de trois types de liquides ioniques à base de phosphonium /ammonium a été étudié. Cette approche s'est avérée supérieure à la micro-flottation conventionnelle appliquée aux mêmes minéraux en termes de sélectivité. De même, il a été démontré que ce procédé peut être appliqué avec efficacité sur une large gamme de pH (4-9) et pour des particules finement broyées (-105 μm) pour fins de valorisation des minéraux de terres rares. Il a été révélé que l'interaction du liquide ionique sur les surfaces minérales se produisait en phase aqueuse, en phase aqueuse-organique et également en phase organique favorisant ainsi l'activation des minéraux et la séparation subséquente.
Dans le troisième cas d'enrichissement des minéraux de terres rares, le potentiel de séparation dans un mélange de deux liquides ioniques immiscibles a enfin été exploré. Un liquide ionique à base de phosphonium / ammonium comme phase dispersées sous forme de gouttelettes et trois liquides ioniques différents faisaint office de phase continue ont été utilisés pour évaluer cette nouvelle approche d'enrichissement des minéraux d’éléments de terres rares. Ce processus s’est avéré très prometteur en tant qu'alternative d'enrichissement des minéraux à base d’éléments de terres rares comparativment aux deux approches précédentes en termes de sélectivité et de récupération des éléments de terres rares.
Enfin dans la dernière partie de la thèse, des simulations de type chimie quantique basée sur la théorie de la densité fonctionnelle (DFT) ont été mises en oeuvre pour proposer une méthode de classification reposant sur la stabilité de la formation de complexes à partir de trente différents liquides ioniques à base de phosphonium aidant à l'extraction par solvant des éléments de terres rares solvatés. Cette étude a d'une part montré que les liquides ioniques choisis peuvent être appliqués plus efficacement pour l'extraction par solvant des éléments de terres rares contenus dans les solutions aqueuses après digestion acide des minéraux
par l’acide nitrique ou chlorhydrique plus que lors d’un recours à l'acide sulfurique. Il a ensuite été démontré que les groupements anioniques des liquides ioniques testés sont capables de former directement des liaisons covalentes par complexation avec les éléments des terres rares solvatés. Au contraire, les interactions des groupements cationiques ont été plus faibles se résumant à des interactions de sphère externe par rapport au complexes formés.
Ce travail de recherche a permis d’explorer la faisabilité dans l'application des liquides ioniques à base de phosphonium au traitement des minéraux et des métaux de terres rares. Les résultats obtenus durant cette étude pourront contribuer à une meilleure compréhension de l’apport des liquides ioniques au secteur des industries des terres rares afin en ayant pour cible l'amélioration de l'efficacité des procédés d'enrichissement des minéraux et d'extraction par solvant des éléments de terres rares dissous.
Abstract
This Ph.D. thesis examines the application of phosphonium/ammonium based ionic liquids in the beneficiation of rare earth element bearing minerals. It has been divided into four main parts. Firstly, the use of phosphonium/ammonium based ionic liquids in three different approaches for rare earth element minerals beneficiation has been explored. These three processes were examined for actual rare earth elements bearing complex ore from Niobec deposit as well as for its constitutive model minerals consisting of monazite and bastnäsite associated with other gangue minerals. Subsequently, application of phosphonium based ionic liquids in rare earth elements solvent extraction has been studied from quantum-chemistry point of view.
In the first process in rare earth element minerals beneficiation, application of a phosphonium/ammonium based ionic liquid as an aqueous collector for froth flotation of rare earth element minerals was studied. In this study, the ionic liquid revealed superior performance to recover rare earth elements bearing minerals as compared with industrial hydroxamic acid collectors. The ionic liquid anionic and cationic moieties interactions during rare earth element minerals flotation were rationalized in terms of an inner synergistic pathway, meaning that the uptake of both cationic and anionic moieties through ionic liquid collector adsorption occurred altogether.
In the second process in rare earth element minerals beneficiation, the potential of liquid-liquid mineral separation mediated by means of three types of phosphonium/ammonium based ionic liquids to beneficiate rare earth elements bearing minerals was studied. This process was found to outperform micro-flotation of the same minerals in terms of selectivity. Likewise, it was shown that this process can be effectively applied over a wide range of pH (4-9) and for fine particle sizes (-105 µm) in rare earth element minerals beneficiation. Interaction of the ionic liquid on the mineral surfaces occurred in aqueous phase, aqueous-organic phases interface and also in the aqueous-organic phase thereby promoting minerals activation and next separation.
In the third process in rare earth element minerals beneficiation, the potential of ionic liquid-ionic liquid mineral separation process as a novel ionic liquid-based system to beneficiate rare earth elements bearing minerals was investigated. A phosphonium/ammonium based ionic liquid as droplet phase and three different ionic liquids as continuous phase were used to assess this approach of beneficiation of rare earth elements bearing minerals. This process revealed high potential, as an alternative, to beneficiate rare earth elements bearing ore as it even outperformed the two previous processes in terms of selectivity and rare earth elements recovery.
In the last part of this thesis, quantum chemistry simulations based on DFT have been undertaken to rank the complex-forming ability of thirty different phosphonium based ionic liquids in solvent extraction of rare earth elements. This study firstly indicated that phosphonium based ionic liquids can be applied more effectively for solvent extraction of rare earth elements in pregnant solutions resulting from nitric and hydrochloride acids leaching process, and less by means of sulfuric acid leaching. It was also demonstrated that while anionic moieties of phosphonium based ionic liquids are able to make directly covalent bonds during complexation with rare earth elements, their cationic moieties can be involved in complexation through outer-sphere interactions.
The implications of this research work include new insights towards application of phosphonium based ionic liquids into mineral and metal processing especially rare earth elements processing. Finding from this work can contribute to the rare earth industry in order to improve efficiency of mineral beneficiation and solvent extraction processes.
Table of contents
Résumé...iii Abstract... v Table of contents...vii List of tables...xii List of figures...xiii Acknowledgements... xx Foreword...xxiChapter 1: Introduction- State of the Art and Objectives ... 1
1.1. State of Technology ... 2
1.2. Rare Earth Elements... 3
1.2.1. Natural Sources, Supply and Possible Problems... 3
1.2.2. Specific Properties of Rare Earth Elements ... 4
1.3. Rare Earth Bearing Minerals Beneficiation ... 6
1.3.1. Rare Earth Bearing Minerals Flotation Difficulties ... 6
1.3.2. Surface Chemistry of Rare Earth Bearing Minerals...7
1.3.3. Collectors in Rare Earth Bearing Minerals Flotation... 8
1.3.4. Depressants in Rare Earth Bearing Minerals Flotation... 10
1.3.5. Effect of Temperature in Rare Earth Bearing Minerals Flotation... 11
1.3.6. Research Opportunities in Rare Earth Bearing Minerals Flotation... 11
1.4. Leaching of Rare Earth Bearing Minerals ... 11
1.4.1. Acid Treatment of Rare Earth Bearing Minerals ... 11
1.4.2. Alkali Treatment of Rare Earth Bearing Minerals... 14
1.4.3. Chlorination of Rare Earth Bearing Minerals ... 15
1.4.4. Additional remarks... 16
1.4.5. Research Opportunities in Rare Earth Bearing Minerals Leaching ... 18
1.5. Solvent Extraction of Rare Earth Elements ... 18
1.5.1. Research Opportunities in Rare Earth Elements Solvent Extraction ... 20
1.6. Ionic Liquids ... 20
1.7. Ionic Liquids in the Context of Minerals and Metals Processing ... 22
1.7.1. Application of Ionic Liquids in Minerals Flotation ... 22
1.7.3. Application of ILs in Solvent Extraction ... 26
1.8. Future State and Market of Ionic Liquids in Industry... 28
1.9. Niobec Deposit... 29
1.10. Scientific Problem and Objectives of This Ph.D. Project ... 30
1.11. The Strategy Adopted to This Ph.D. Project... 30
1.12. Contributions of This Ph.D. Thesis... 33
1.13. References... 34
Chapter 2: Application of Phosphonium/Ammonium Based Ionic Liquids in Froth Flotation Process. 44 Résumé... 45
Abstract... 46
2.1. Introduction... 47
2.2. Materials & Methods ... 50
2.2.1. Minerals ... 50
2.2.2. Chemical Reagents... 51
2.2.3. Micro-flotation Tests... 52
2.2.4. Zeta Potential ... 53
2.2.5. Infrared Spectroscopy ... 53
2.2.6. X-ray Photoelectron Spectroscopy... 53
2.3. Results and Discussion... 54
2.3.1. Micro-Flotation Tests... 54
2.3.2. Zeta Potential ... 57
2.3.3. Infrared Spectroscopy ... 58
2.3.4. X-ray Photoelectron Spectroscopy... 60
2.4 . IL Collector Conformation on Bastnäsite and Monazite Surfaces... 65
2.5. Conclusion ... 67
2.6. Acknowledgments... 68
2.7. References... 68
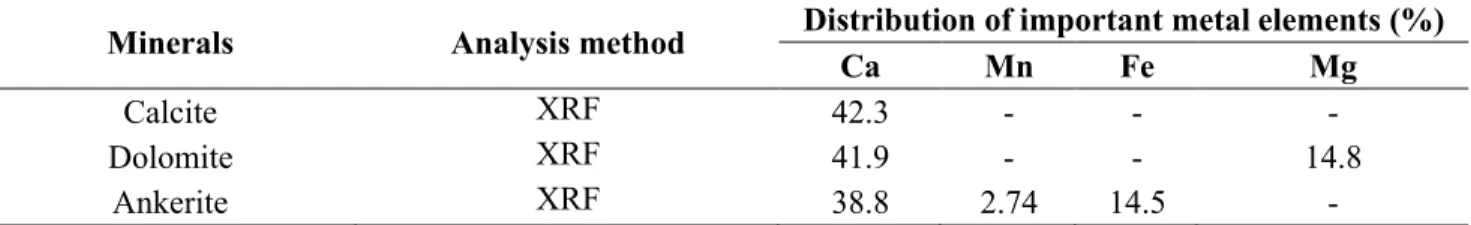
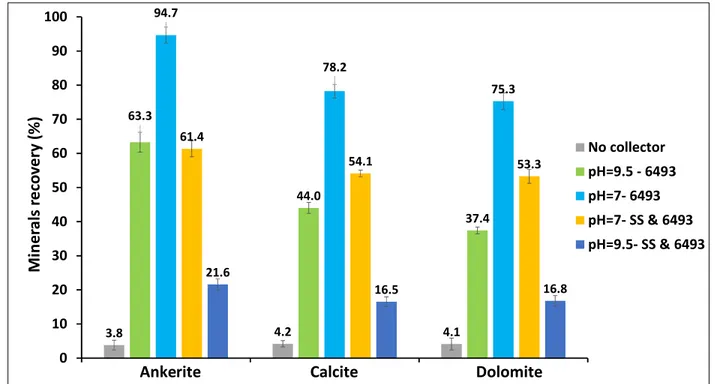
Chapter 3: Surface interactions and flotation behavior of calcite, dolomite and ankerite with alkyl hydroxamic acid bearing collector and sodium silicate ... 73
Résumé:... 74
Abstract... 75
3.1. Introduction... 76
3.2. Materials & Methods ... 77
3.2.2. Chemical Reagents... 78
3.2.3. Micro-Flotation Tests... 78
3.2.4. Zeta Potential Measurements... 79
3.2.5. Infrared Spectroscopy ... 79
3.2.6. Density Functional Theory Simulation ... 80
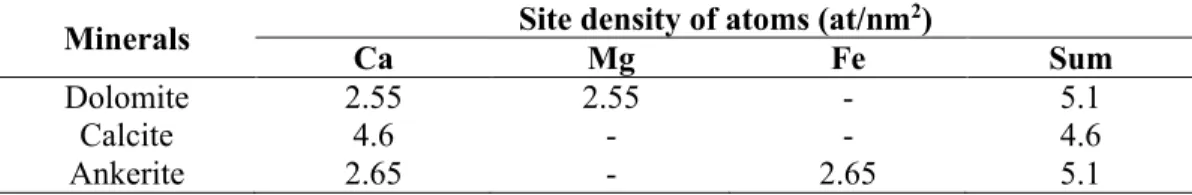
3.3. Results & Discussion ... 81
3.3.1. Micro-flotation Experiments... 81
3.3.2. Zeta Potential Measurements... 83
3.3.3. Infrared Spectroscopy ... 84
3.3.4. Density Functional Theory Simulation ... 86
3.4. Conclusion ... 95
3.5. Acknowledgments... 96
3.6. References... 96
Chapter 4: Application of Phosphonium/Ammonium Based Ionic Liquids in Liquid-Liquid Mineral Separation Process ... 100
Résumé... 101
Abstract... 102
4.1. Introduction... 103
4.2. Experimental ... 104
4.2.1. Minerals, Characterization and Preparation... 104
4.2.2. Chemicals and Reagents ... 106
4.2.3. Mineral Separation... 106
4.2.4. Zeta Potential Determination ... 108
4.2.5. FTIR Spectra of Aqueous and Organic Liquids & Solid Samples... 108
4.3. Theoretical ... 109
4.3.1. Van der Waals and Electrostatic Interfacial Interactions ... 109
4.3.2. Quantum Mechanical Simulation... 111
4.5. Results and Discussion... 112
4.5.1. Liquid-Liquid Mineral Separation of Model Minerals versus Operating Conditions... 112
4.5.2. Liquid-Liquid Mineral Separation of Model Minerals versus IL Cation/Anion... 115
4.5.3. Liquid-Liquid Mineral Separation versus Flotation REM Recovery from Niobec Ore 115 4.5.4. Zeta Potential ... 117
4.5.5. Van der Waals and Electrostatic Interfacial Interactions ... 119
4.5.7. Quantum Mechanical Simulation... 128
4.5.8. Role of pH on REE Mineral Separation... 132
4.6. Conclusions... 134
4.7. Acknowledgments... 135
4.8. References... 135
Chapter 5: Application of Phosphonium/Ammonium Based Ionic Liquids in Ionic Liquid- Ionic Liquid Mineral Separation Process... 141
Résumé... 142
Abstract... 143
5.1. Introduction... 144
5.2. Materials and Methods... 145
5.2.1. Minerals, Characterization and Preparation... 145
5.2.2. Ionic Liquids ... 147
5.2.3. IL-IL Mineral Separation ... 149
5.2.4. Infrared Spectroscopy ... 150
5.2.5. X-ray Photoelectron Spectroscopy... 150
5.2.6. Atomic Force Microscopy ... 150
5.2.7. Density Functional Theory Simulation ... 151
5.3. Results and Discussion... 152
5.3.1. IL-IL Mineral Separation ... 152
5.3.2. Ionic Liquid-Mineral Interactions... 154
5.3.3. Ionic Liquid Configuration on Calcite Surface... 162
5.4. Conclusion ... 166
5.5. Acknowledgments... 166
5.6. References... 166
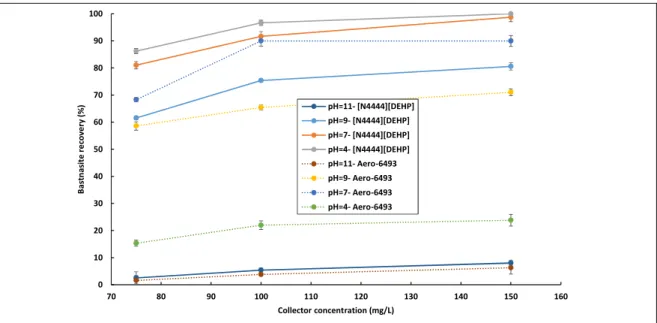
Chapter 6: Towards Understanding Behavior of Bifunctional Phosphonium Based Ionic Liquids in Solvent Extraction of Rare Earth Elements: A Quantum Chemistry Study... 172
Résumé... 173
Abstract... 174
6.1. Introduction... 175
6.2. Background ... 176
6.2.1. Rationale for Ionic Liquids selection in REEs Solvent Extraction ... 176
6.2.2. Quantum Mechanical Calculations ... 178
6.3.1. Solvation of REE Species in Aqueous Environment ... 179
6.3.2. Solvation of Ionic Liquids in Organic Environment... 181
6.3.3. Ionic Liquids- REE Species Complexation Energies... 187
6.3.4. Ionic liquids- REE Species Complexation Mechanism ... 192
6.4. Conclusion ... 193
6.5. Acknowledgments... 194
6.6. References... 194
Chapter 7: Conclusion and Recommendations for Future Work... 197
7.1. Key Contributions and Conclusions of Thesis... 198
7.2. Recommendations for Future Work... 200
Appendix A... 202
A.1. Rationale for Ionic Liquid Selection ... 202
A.2. References... 204
Appendix B ... 205
Appendix C ... 206
C.1. FTIR Study on Interaction of Continuous IL Phase with Minerals... 206
C.2. DFT Simulation Study on Interaction of Continuous IL Phase with Minerals ... 206
Appendix D... 213
List of tables
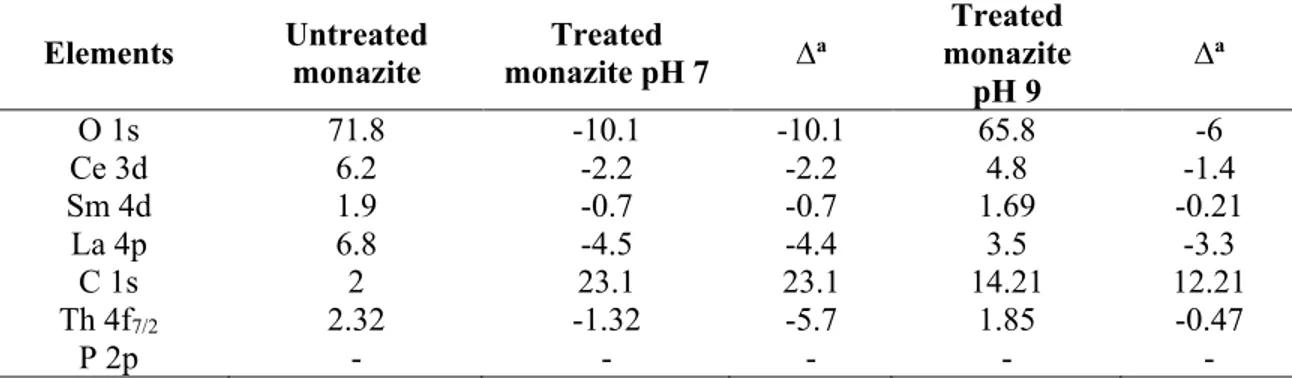
Table 1-1: The average price of selected rare earth oxides (March 2015) [14]……….…3 Table 1-2: Summary of leaching technologies in production of REEs from monazite and bastnäsite [87]………...17 Table 2-1: Surface composition of [N4444][DEHP] from XPS……….……61
Table 2-2: Surface composition (%) of some elements for bare and IL treated (pH 7, 9) bastnäsite
(atreated – bare)……….………61
Table 2-3: Group contributions (relative to C1s) of carbon on bastnäsite surface before and after IL treatment at pH 7 and 9 (atreated – bare)………...62
Table 2-4: Surface composition (%) of some elements for bare and IL treated (pH 7, 9) monazite (atreated
– bare)………...62 Table 2-5: Surface composition (%) of some elements for bare and IL treated (pH 7, 9) calcite (atreated
– bare)………...62 Table 3-1: Concentration of main metal elements of model mineral samples obtained from XRF……...78 Table 3-2: Surface area of all model mineral samples used in flotation and FTIR………78 Table 3-3: Effect of pH on performance of sodium silicate through flotation of calcite, dolomite and ankerite……….82 Table 3-4: Site density of the abundant metal atoms on the surface of minerals (slab (104)………….….90 Table 3-5: Energy of mineral-collector interaction in both bridged and monodentate conformation (slab (104)……….93 Table 3-6: Interaction energy of SiO(OH)3-and Si(OH)4 on the mineral surfaces (slab (104))………….94
Table 4-1: BET surface area of mineral samples used in ζ potential, FTIR and liquid-liquid minerals separation………...105 Table 4-2: Calculated maximum repulsive interaction energies, (VA+ VE)max/kT, between the individual
model minerals and n-hexane droplets in aqueous phase………122 Table 5-1: Concentration of main metal elements of model mineral samples obtained from XRF and ICP-MS………..146 Table 5-2: Specific surface area of model mineral samples used in FTIR and XPS analyses, and IL-IL mineral separation………..147 Table 5-3: Chemical structure and physical properties of ionic liquids tested in current study…….…..148 Table 5-4: Comparison between new method and previous methods in terms of average recovery difference of REE and gangue (calcite and dolomite)* minerals………154 Table 5-5: Surface composition of [N4444][DEHP] from XPS……….………158
Table 5-6: Surface composition (%) of some elements for bare and IL-treated bastnäsite, monazite and ankerite………...159 Table 5-7: Group contributions (relative to C1s) of carbon on mineral surfaces and BE shift of oxygen in various species before and after IL treatment………..160 Table 5-8: Computed interaction energies between calcite [104] slab surface and droplet and continuous IL phases………165 Table 6-1: Mulliken charge analysis of solvated REE chlorides in aqueous phase ………….…………180 Table 6-2: Mulliken charge analysis of solvated REE nitrates in aqueous phase ………...181 Table 6-3: Mulliken charge analysis of solvated REE sulfates in aqueous phase……….………...181
List of figures
Fig.1-1: Most important countries in rare earth production (2014) [14]………...4
Fig.1-2: Reduction in ionic radius of REEs by increasing their atomic number [10]……….…………...5
Fig.1-3: Relationship between atomic number of lanthanides and thermodynamic functions (Kex extraction equilibrium constant, ΔH enthalpy variation, ΔZ0r free energy variation, and ΔS0r entropy variation) of an extraction reaction from a system consisting of 2-ethyl hexyl mono(2-ethyl hexyl)ester phosphinate in a dodecane solution [10]…………...………..6
Fig.1-4: General acid roasting and leaching process for bastnäsite and monazite [67]…….……….12
Fig.1-5: General HCl leaching process for bastnäsite [67]……….…………...13
Fig.1-6: Monazite processing by alkali treatment [9]………...15
Fig.1-7: Typical schematic of SX process [9]………...18
Fig.1-8: Current and future applications of ILs [101]………...21
Fig.1-9: Overall dissolution reaction of bastnäsite in ILs system [156]………...25
Fig.1-10: An example of the three-path model for metal ion extraction [107]………...26
Fig.1-11: Proposed structure of [A336][P204] and its coordination environment with Eu(III) [127].………...………...28
Fig. 1-12: Technology readiness levels (TRL) of IL-based technologies [186]………...29
Fig. 1-13: A schematic illustration of the adopted doctoral.……….………...32
Fig. 2-1: X-ray powder diffractograms of Niobec ore, monazite, bastnäsite, calcite, dolomite and quartz samples………...51
Fig. 2-2: Mini-flotation column setup to study recovery of bare and IL-treated REE minerals and ore 1. air inlet 2. porous disk gas distributor 3. air bubbles 4. pH-meter electrode 5. solids inventory 6. liquid make up injection 7. liquid outlet 8. air outlet………...52
Fig. 2-3: Micro-flotation tests of Niobec ore with hydroxamic acid-containing collectors and [N4444][DEHP]: a) recovery of Nd+Ce+La, and b) enrichment ratio of Nd+Ce+La (pH 9, IL and collector concentration = 100 mg/L, sodium silicate concentration = 10 mg/L)………..54
Fig. 2-4: Micro-flotation tests of model minerals with Aero-6493 and [N4444][DEHP] (pH 9, IL and collector concentration = 100 mg/L)……….55
Fig. 2-5: Effect of pH and collector concentration on bastnäsite flotation………56
Fig. 2-6: Effect of pH and collector concentration on monazite flotation……….……….56
Fig. 2-7: Zeta potential measurements for bastnäsite, monazite and calcite without (untreated) and with (treated) [N4444][DEHP]………57
Fig. 2-8: FTIR spectra of [N4444][DEHP] (a), model bastnäsite before IL treatment (b) and after IL treatment with [N4444][DEHP] at pH 7 (c) and pH 9 (d)………...….58
Fig. 2-9: FTIR spectra of [N4444][DEHP] (a), model monazite before IL treatment (b) and after IL treatment with [N4444][DEHP] at pH 7 (c) and pH 9 (d)……….…..………….59
Fig. 2-10: FTIR spectra of [N4444][DEHP] (a), model calcite before IL treatment (b) and after IL treatment with [N4444][DEHP] at pH 7 (c) and pH 9 (d)………60
Fig. 2-11: XPS survey spectrum of [N4444][DEHP]……….………..61
Fig 2-12: High-resolution XP P 2p core level spectra along with fitted features for pure [N4444][DEHP] (a), bastnäsite samples recovered after flotation after adsorption of [N4444][DEHP] at pH 9 (b) and pH 7 (c). Dotted vertical lines correspond to fitted BE peak positions………...64
Fig. 2-13: High-resolution XP N 1s core level spectra along with fitted features for as-received model bastnäsite sample (a), and bastnäsite samples recovered after flotation after adsorption of [N4444][DEHP]
at pH 9 (b) and pH 7 (c). Dotted vertical lines correspond to fitted BE peak positions………..65 Fig. 2-14: Speculated adsorption pathway of [N4444][DEHP] on bastnäsite and monazite surfaces at 7 <
pH < 9………...66 Fig. 2-15: Speculated interactions of IL anionic and cationic moieties on the bastnäsite and monazite surfaces at 7<pH<9: (a) electrostatic interaction between IL cation and mineral surface, (b) chemisorption of IL anion on mineral surface, (c) lateral chain–chain associative interaction between chemisorbed anion and cation due to London dispersion forces between hydrophobic chains………...…….67 Fig. 3-1: Powder X-ray diffraction patterns of model single-minerals including calcite, dolomite and ankerite samples………...77 Fig. 3-2: The results of micro-flotation tests for calcite, dolomite and ankerite using Aero-6493 and sodium silicate (SS) at pH 7 and 9.5………82 Fig. 3-3: Speciation distribution of silicate anions as a function of pH in 0.1 mmol/L (equal to flotation condition) aqueous sodium silicate solution (done by Medusa software, as a chemical equilibrium diagrams software)………...83 Fig. 3-4: Zeta potential measurements values of calcite, dolomite and ankerite for bare and treated minerals with Aero-6493 (a), sodium silicate (SS) (b), sodium silicate (SS) and Aero-6493 (c)………...84 Fig. 3-5: FTIR spectra of Aero-6493 (a), bare ankerite powder (b), treated ankerite with Aero-6493 at pH 7 (c), treated ankerite with 6493 at pH 9.5 (d), treated ankerite with sodium silicate (SS) and Aero-6493 at pH 7 (e)………..…………...85 Fig. 3-6: FTIR spectra of Aero-6493 (a), bare calcite powder (b), treated calcite with Aero-6493 at pH 7 (c), treated calcite with Aero-6493 at pH 9.5 (d), treated calcite with sodium silicate (SS) and Aero-6493 at pH 7 (e)……….85 Fig. 3-7: FTIR spectra of Aero-6493 (a), bare dolomite powder (b), (c) treated dolomite with Aero-6493 at pH 7 (c), treated dolomite with Aero-6493 at pH 9.5 (d), treated dolomite with sodium silicate (SS) and Aero-6493 at pH 7 (e)……….86 Fig. 3-8. The optimized structures of dolomite crystal (a), calcite crystal (b), and ankerite crystal (c): Ca Green, O Red, C Gray, Mg Orange, Fe Blue………..87 Fig. 3-9: The optimized structure of calcite slab (104) from different views (a-c): Ca Green, O Red, C Gray………...88 Fig. 3-10: The optimized structure of dolomite slab (104) from different views (a-c): Ca Green, O Red, C Gray, Mg Orange………88 Fig. 3-11: The structure of ankerite slab (104) before (a-c), and after (d-f) optimization from different views: Ca Green, O Red, C Gray, Fe Blue……….89 Fig. 3-12. DFT optimized structure of octyl hydroxamic acid anion (a), Si(OH)4(b), and SiO(OH)3-(c),
and also Highest occupied molecular orbitals (HOMO) of octyl hydroxamic acid anion (d), Si(OH)4(e),
SiO(OH)3-(f): N Blue, O Red, Si Yellow, C Gray, H White……….………..90
Fig. 3-13: Octyl hydroxamic acid anion adsorption geometries on calcite slab (104) through bridged (a), and monodentate (b) conformations: N Blue, O Red, C Gray, H White, Ca Green………92 Fig. 3-14: Octyl hydroxamic acid anion adsorption geometries on dolomite slab (104) through bridged (a), and monodentate (b) conformations: N Blue, O Red, C Gray, H White, Ca Green, Mg Orange………..92
Fig. 3-15: Octyl hydroxamic acid anion adsorption geometries on ankerite slab (104) through bridged (a), and monodentate (b) conformations: N Blue, O Red, C Gray, H White, Ca Green, Fe Blue………..93 Fig. 3-16: Si(OH)4adsorption geometries on ankerite slab (104) (a), calcite slab (104) (b), and dolomite
slab (104) (c) and also SiO(OH)3-adsorption geometries on ankerite slab (104) (d), calcite slab (104) (e),
and dolomite slab (104) (f): N Blue, O Red, C Gray, H White, Ca Green, Fe Blue, Mg Orange………..95 Fig 4-1: Powder X-ray diffraction patterns of Niobec ore, and of model single-mineral bastnäsite, monazite, and calcite and dolomite samples………..105 Fig 4-2: Molecular structures of [N4444][DEHP], [N2222][diOctP] and [N2222][DEHP] ionic liquids.…106
Fig 4-3: Illustration of liquid-liquid mineral separation (a) IL organic phase ① & aqueous phase + mineral ②; (b) 3-phase pulp under mixing ③; (c) IL organic phase + oleophilic minerals ④ & aqueous phase + hydrophilic mineral leftover ⑤……….107 Fig 4-4: Liquid-liquid mineral separation of coarse bastnäsite with n-hexane (a) and kerosene (b), and of fine bastnäsite with n-hexane (c) and kerosene (d). Standard deviations on recovery shown as error bars……….113 Fig 4-5: Liquid-liquid mineral separation of coarse monazite with n-hexane (a) and kerosene (b), and of fine monazite with n-hexane (c) and kerosene (d). Standard deviations on recovery shown as error bars……….113 Fig 4-6: Liquid-liquid mineral separation of coarse calcite with n-hexane (a) and kerosene (b), and of fine calcite with n-hexane (c) and kerosene (d). Standard deviations on recovery shown as error bars……….114 Fig 4-7: Liquid-liquid mineral separation of coarse dolomite with n-hexane (a) and kerosene (b), and of fine dolomite with n-hexane (c) and kerosene (d). Standard deviations on recovery shown as error bars……….114 Fig 4-8: Incidence on REM recovery of IL anionic and cationic moieties: coarse and fine bastnäsite (a), coarse and fine monazite (b) using 150 ppm [N2222][DEHP], [N2222][diOctp] and [N4444][DEHP] ionic
liquids. Standard deviations on recovery shown as error bars……….115 Fig 4-9: Liquid-liquid mineral separation for Niobec ore fine and coarse fractions using 100 ppm [N4444][DEHP] in n-hexane as a function of aqueous pulp pH : La+Ce+Nd recovery in concentrates (a)
and mass recovery of ore displaced from aqueous to organic phase (b); REE enrichment ratio in concentrates (c); comparison of liquid-liquid mineral separation versus flotation enrichment ratios for hydroxamic acid (Aero 6493 & Aero 6849) and [N4444][DEHP] aqueous collectors at 100 pm concentrations and pH ~ 9 (d)……….117 Fig 4-10: Zeta potential evolution with pH of (a) aqueous suspensions of model single-mineral bastnäsite, monazite, dolomite and calcite powders at 0.1 wt.%, (b) emulsion droplets of IL-free kerosene and kerosene containing 75, 100 and 150 ppm [N4444][DEHP], and c) of emulsion droplets of IL-free n-hexane
and n-hexane containing 75, 100 and 150 ppm [N4444][DEHP]………...118
Fig 4-11: Speculative mechanism based on zeta potential measurements and FTIR vibrational spectra to interpret transfer into aqueous solution of (a) [N4444][DEHP] anion for pH < 2 and (b) [N4444][DEHP]
cation for pH > 2……….119 Fig 4-12. DLVO-estimated total interaction energy (Eqs.1,2) between n-hexane and (a) bastnäsite, (b) monazite, (c) dolomite, (d) calcite in the pH range from 2 to 11. Energy normalized by thermal agitation
kT and particle-droplet separation distance expressed as fractions of the Debye characteristic length, k-1
= (εrε0RT/2∙103/F2)0.5I-0.5for 1µm colloidal mineral particles……….121
Fig 4-13: Evolution of sign (VA VE) as a function of separation distance between n-hexane and (a)
bastnäsite, (b) monazite, (c) dolomite, (d) calcite in the pH range from 2 to 11………...122 Fig 4-14: FTIR spectra of IL-free aqueous solutions at pH 1-11 (a) and of residual aqueous solutions at pH 1 (b), pH 4 (c), pH 7 (d), pH 9 (e), pH 11 (f) according to modality A (Section 4.2.5) following aqueous solution contacting with 200 ppm [N4444][DEHP]/n-hexane……….123
Fig 4-15: FTIR spectra of [N4444][DEHP] (a), bare bastnäsite powder (b), and treated bastnäsite pulped
in residual [N4444][DEHP]/n-hexane solutions according to modality B and pH = 1-11 (Section 4.2.5)
(c)………...125 Fig 4-16: FTIR spectra of [N4444][DEHP] (a), bare monazite powder (b), and treated monazite pulped in
residual [N4444][DEHP]/n-hexane solutions according to modality B and pH = 1-11 (Section 4.2.5)
(c)………...125 Fig 4-17: FTIR spectra of [N4444][DEHP] (a), bare calcite powder (b), and treated calcite pulped in
residual [N4444][DEHP]/n-hexane solutions according to modality B and pH = 1-11 (Section 4.2.5)
(c)………...126 Fig 4-18: FTIR spectra of [N4444][DEHP] (a), bare dolomite powder (b), and treated dolomite pulped in
residual [N4444][DEHP]/n-hexane solutions according to modality B and pH = 1-11 (Section 4.2.5)
(c)………...126 Fig 4-19: FTIR spectra of pure [N4444][DEHP] (a), bare bastnäsite (b) and bastnäsite pulped with residual
aqueous solution at pH 1 (c), pH 4 (d), pH 9 (e), pH 11 (f) according to modality C (Section 4.2.5) following aqueous solution contacting with 200 ppm [N4444][DEHP]/n-hexane………127
Fig 4-20: FTIR spectra of pure [N4444][DEHP] (a), bare monazite (b) and monazite pulped with residual
aqueous solution at pH 1 (c), pH 4 (d), pH 9 (e), pH 11 (f) according to modality C (Section 4.2.5) following aqueous solution contacting with 200 ppm [N4444][DEHP]/n-hexane………128
Fig 4-21: DFT optimized structure of (a) Ce(H2O)103+ , (b) Ca(H2O)82+ and (c) Highest occupied
molecular orbitals (HOMO) of [N4444][DEHP]: Ca Green, Ce Brown, N Blue, O Red, P Purple,
C Gray, H White………...130 Fig 4-22: DFT optimized structures resulting from the interaction of (a) Ce(H2O)103+and [N4444][DEHP]
, (b) Ca(H2O)82+and [N4444][DEHP] under water COSMO assumption: Ca Green, Ce Brown, N
Blue, O Red, P Purple, C Gray, H White……….131 Fig 4-23: DFT optimized structures resulting from the interaction of (a) Ce(H2O)103+and [N4444][DEHP]
, (b) Ca(H2O)82+and [N4444][DEHP] under n-hexane COSMO assumption: Ca Green, Ce Brown, N
Blue, O Red, P Purple, C Gray, H White………..132 Fig 4-24: Portrayal of the IL-RE mineral interactions over pH 4-9 (a) aqueous activation (1) of RE minerals followed then by hydrophobic interaction (2) with polar solvent, (b) engulfment into non-polar solvent of RE mineral involving covalent bonds with IL anion and weak Coulombic interactions with ion-par IL cation, (c) interaction between IL anionic moiety and minerals surface at non-polar solvent/water interface………...134 Fig. 5-1: Powder X-ray diffraction patterns of Niobec complex REE ore and model single-mineral bastnäsite, monazite, ankerite, calcite and dolomite samples……….…….146 Fig. 5-2: Visualization of phase separation after IL-IL pulp mixing………...149 Fig. 5-3: IL-IL mineral separation results for recovery of monazite, bastnäsite, calcite, ankerite and dolomite……….……….153
Fig. 5-4: FTIR spectra of [N4444][DEHP] (a), bare bastnäsite powder (b), and treated bastnäsite with pure
[N4444][DEHP] (c)………...156
Fig. 5-5: Force versus distance profile for an AFM tip approaching (blue) and retracting from (red) a bastnäsite surface in [N4444][DEHP] (a), monazite surface in [N4444][DEHP] (b), ankerite surface in
[N4444][DEHP] (c) and calcite surface in [N4444][DEHP] (d)………..157
Fig. 5-6: Ce 3d spectra for untreated bastnäsite (a, b), and for treated bastnäsite with [N4444][DEHP] (c,
d)………161 Fig. 5-7: Ce 3d spectra for untreated monazite (a, b), and for treated monazite with [N4444][DEHP] (c, d)
and also La 3d spectra for untreated monazite (e), and for treated monazite with [N4444][DEHP] (f)…..161
Fig. 5-8: Ca 2p spectra for untreated ankerite (a), and for treated ankerite with [N4444][DEHP] (b) and
also Fe 2p spectra for untreated ankerite (c), and for treated ankerite with [N4444][DEHP] (d)…………162
Fig. 5-9: DFT optimized structure of (a) [N4444][DEHP], and (b) Highest occupied molecular orbitals
(HOMO) of [N4444][DEHP]: N Blue, O Red, P Purple, C Gray, H White……….163
Fig. 5-10: The structure of calcite crystal (a), and calcite slab [104] (b): Ca Green, O Red, C Gray, H White………...164 Fig. 5-11: [N4444][DEHP] adsorption geometries on calcite slab [104] from different views: N Blue, O
Red, P Purple, C Gray, H White, Ca Green………..165 Fig 6-1: Complex formation at interfacial reaction site between IL and REE species during solvent extraction and shuttling of REE from aqueous to organic phase. ………...……….………….177 Fig 6-2: DFT optimized structure of (a) LaCl3(H2O)6 , (b) La(NO3)3(H2O)3, and (c) [La(SO4)3(H2O)3]3-as
representative examples of chloride-, nitrate- and sulfate-bearing REE aqua-complexes………..179 Fig 6-3: DFT optimized structure of (a) [DEHP][P66614], (b) [DEHP][C6mim], (c) [DEHP][C6mpyr], (d)
[DEHP][N2222], (e) [DEHP][N4444], (f) [DEHP][N8888], with corresponding HOMOs and LUMO…….183
Fig 6-4: DFT optimized structure of (a) [EHEHP][P66614], (b) [EHEHP][C6mim], (c) [EHEHP][C6mpyr],
(d) [EHEHP][N2222], (e) [EHEHP][N4444], (f) [EHEHP][N8888], with corresponding HOMOs and
LUMOs………..186 Fig 6-5: DFT optimized structure of (a) [C272][P66614], (b) [C272][C6mim], (c) [C272][C6mpyr], (d)
[C272][N2222], (e) [C272][N4444], (f) [C272][N8888], with corresponding HOMOs and LUMOs……….186
Fig 6-6: DFT optimized structure of (a) [C302][P66614], (b) [C302][C6mim], (c) [C302][C6mpyr], (d)
[C302][N2222], (e) [C302][N4444], (f) [C302][N8888], with corresponding HOMOs and LUMOs…….…187
Fig 6-7: DFT optimized structure of (a) [C301][P66614], (b) [C301][C6mim], (c) [C301][C6mpyr], (d)
[C301][N2222], (e) [C301][N4444], (f) [C301][N8888], with corresponding HOMOs and LUMO………..187
Fig 6-8: Evolution of the IL-REE interaction energy during the complexation of light and heavy REE aqua-complex species as a function of IL cations in the case of [DEHP] anionic moiety: (a) REEsCl3(H2O)6, (b) REEs(NO3)3(H2O)3, and (c) [REEs(SO4)3(H2O)3]3-……….…….189
Fig 6-9: Evolution of the IL-REE interaction energy during the complexation of light and heavy REE aqua-complex species as a function of IL cations in the case of [EHEHP] anionic moiety: (a) REEsCl3(H2O)6, (b) REEs(NO3)3(H2O)3, and (c) [REEs(SO4)3(H2O)3]3-. ………189
Fig 6-10: Evolution of the IL-REE interaction energy during the complexation of light and heavy REE aqua-complex species as a function of IL cations in the case of [C272] anionic moiety: (a) REEsCl3(H2O)6, (b) REEs(NO3)3(H2O)3, and (c) [REEs(SO4)3(H2O)3]3-………..……190
Fig 6-11: Evolution of the IL-REE interaction energy during the complexation of light and heavy REE aqua-complex species as a function of IL cations in the case of [C302] anionic moiety: (a) REEsCl3(H2O)6, (b) REEs(NO3)3(H2O)3, and (c) [REEs(SO4)3(H2O)3]3-………..…………190
Fig 6-12: Evolution of the IL-REE interaction energy during the complexation of light and heavy REE aqua-complex species as a function of IL cations in the case of [C301] anionic moiety: (a) REEsCl3(H2O)6, (b) REEs(NO3)3(H2O)3, and (c) [REEs(SO4)3(H2O)3]3-………...191
Fig 6-13: DFT calculated interaction energies during complexation of the best selected ILs in each IL group with (a) REEsCl3, (b) REEs(NO3)3, and (c) [REEs(SO4)3]3-………...192
Fig 6-14: Schematics of DFT-optimized structures of IL-REE species complex at the aqusous-organic phase interface and inside the organic phase.………..193
“To my mother, my father’s soul and my beautiful and intelligent wife”
“Anyone who genuinely and consistently with both hands look for something, will find it.”
Acknowledgements
I would like to express my sincere thanks and appreciation to all those who have made this thesis possible. My deepest gratitude goes first and foremost to my supervisor, Professor Faïçal Larachi, for giving me the opportunity to undertake this Ph.D. and for his scientific guidance during these years. It was an honor for me to be a part of his research group.
I acknowledge all the people at Laval University, especially Jerome Nöel for his outstanding help in the fabrication and modification of different experimental setups. Special thanks to Professor Fathi Habashi and Professor Nadir Belkhiter for their helps and supports to find this Ph.D. position. I also gratefully acknowledged Dr. Alain Adnot for his help in interpreting the minerals XP spectra, and Dr. Mohammad Latifi for leading the synergy initiatives in the project with our RTIL-REE group. I am also very grateful to Madam Denise Baillargeon for her endless weekly effort during last years to help me to improve my French and also for her emotional supports regarding my Ph.D. works.
I would like to thank for the financial support I received which enabled me to do this PhD. I am grateful for financial support from the Natural Sciences and Engineering Research Council (NSERC) through its Cooperative Research & Development grants program and from the supporting partner Niobec inc. I am also indebted to Compute Canada for the HPC platform without which the present DFT simulations would not have been possible.
My sincere gratefulness to all my friends, an inspiring group of people especially Ali Entezari-Zarandi, Shahab Boroun, Amir Motamed, Olivier Gravel, Muhammad Khalid, Diana Aksenova, and also my best friend in Iran, Ali Hassankhani.
I would like to acknowledge the support of my mother, my sisters, Somayeh, Tahmineh, Shabnam, my brothers, Koorosh and Arash, my sister in law, Marzieh, and my brother in law, Dariush, for their endless love and support during the time of this study and also for strongly encouraging me to pursue my Ph.D. in abroad. Moreover, I would like to acknowledge my kind father, who is not between us for several years, but I cannot forget his support and influence in my life.
Finally, I would like to express my gratitude to my love, my best friend, and my beautiful and intelligent wife, Foroogh, for her helps, supports and kindness during last four years in my life. She has been always beside me to cross over my Ph.D. journey at Laval.
Foreword
This thesis is composed of eight chapters and presented as articles in insertion form. The problem statement, objectives, and also adopted strategy and general methodology are presented in Chapter 1. Chapter 1 is also a critical review focusing on REEs industry, REE mineral processing and application of ILs in minerals and metals processing with special regards to REE minerals. Chapter 1 is originally written by Dariush Azizi and have never been published before. Chapters 2, 3, 4, and 5 present the results of this project in the form of scientific articles. Chapter 6 has been recently submitted into Journal of Molecular Liquids and it is under revision. Chapter 7 highlights the general conclusions and recommendations for future studies. Chapter 7 is also originally written by Dariush Azizi and have never been published before. This project was supervised by Prof. Faïçal Larachi.
Chapter 2: Ionic-liquid collectors for rare-earth minerals flotation—Case of tetrabutylammonium
bis(2-ethylhexyl)-phosphate for monazite and bastnäsite recovery Authors: Dariush Azizi a, Faïçal Larachi a∗, Mohammad Latifi b
aDepartment of Chemical Engineering, Université Laval, 1065 Avenue de la Médecine, Québec, Québec
G1V 0A6, Canada.
bProcess Engineering Advanced Research Lab (PEARL), Department of Chemical Engineering, École
Polytechnique de Montréal, Montréal, Québec, Canada.
Journal: Colloids and Surfaces A: Physicochem. Eng. Aspects 506 (2016), 74–86. Elsevier. DOI: 10.1016/j.colsurfa.2016.06.011
All experiment measurements and analysis along with the paper have been written and presented by Dariush Azizi. The scientific revision was done by Prof. Faïçal Larachi and Dr. Mohammad Latifi.
Appendix A is also related to this chapter as supplementary information of corresponding paper.
Chapter 3: Surface interactions and flotation behavior of calcite, dolomite and ankerite with alkyl
hydroxamic acid bearing collector and sodium silicate Authors: Dariush Azizi a, Faïçal Larachi a∗
aDepartment of Chemical Engineering, Université Laval, 1065 Avenue de la Médecine, Québec, Québec
G1V 0A6, Canada.
Journal: Colloids and Surfaces A: Physicochem. Eng. Aspects 537 (2018), 126-138. Elsevier.
All experiment measurements, DFT simulations and analysis along with the paper have been written and presented by Dariush Azizi. The scientific revision was done by Prof. Faïçal Larachi.
The interaction nature of alky hydroxamic acid collector with monazite and bastnäsite was also studied in order to provide further information related to chapter 2. In this regard, another article titled “Hydroxamic
acid interactions with solvated cerium hydroxides in the flotation of monazite and bastnäsite— Experiments and DFT study” co-authored by Dr. Amin Sarvaramini, Dariush Azizi, and Prof.
Faïçal Larachi, and it has been published in journal of Applied Surface Science 387 (2016), 986–995,
Elsevier. DOI: 10.1016/j.apsusc.2016.07.044. However, only its abstract was given in the Appendix B since Dariush Aziz is second author in this article. All experiment measurements and their analysis along with the paper have been written and presented by Dariush Azizi. All DFT simulation was performed and analyzed by Dr. Amin Sarvaramini. Dariush Aziz was also participating to discuss about relationship between DFT and experimental results and also writing the introduction. The scientific revision of this article was done by Prof. Faïçal Larachi.
Chapter 4: Liquid-liquid mineral separation via ionic-liquid complexation of monazite and bastnäsite—
An alternate route for rare-earth mineral beneficiation
Authors: Dariush Azizi a, Amin Sarvaraminia, Faïçal Larachi a∗
aDepartment of Chemical Engineering, Université Laval, 1065 Avenue de la Médecine, Québec, Québec
G1V 0A6, Canada.
Journal: Colloids and Surfaces A: Physicochem. Eng. Aspects 520 (2017) 301–323. Elsevier. DOI: 10.1016/j.colsurfa.2017.01.079
All experiment measurements and their analysis along with the paper have been written and presented by Dariush Azizi. All DFT simulation was performed and analyzed by Dr. Amin Sarvaramini. Dariush Azizi was also participating to discuss about relationship between DFT and experimental results. The scientific revision of this article was done by Prof. Faïçal Larachi.
Chapter 5: Immiscible dual ionic liquid-ionic liquid mineral separation of rare-earth minerals
Authors: Dariush Azizi a, Faïçal Larachi a∗
aDepartment of Chemical Engineering, Université Laval, 1065 Avenue de la Médecine, Québec, Québec
G1V 0A6, Canada.
Journal: Separation and Purification Technology 191 (2018), 340-353. Elsevier. DOI:10.1016/j.seppur.2017.09.061
All experiment measurements, DFT simulations and analysis along with the paper have been written and presented by Dariush Azizi. The scientific revision was done by Prof. Faïçal Larachi.
Appendix C is also related to this chapter as supplementary information of corresponding paper. Chapter 6: Behavior of Bifunctional Phosphonium-Based Ionic Liquids in Solvent Extraction of Rare
Earth Elements - Quantum Chemistry Study Authors: Dariush Azizi a, Faïçal Larachi a∗
aDepartment of Chemical Engineering, Université Laval, 1065 Avenue de la Médecine, Québec, Québec
G1V 0A6, Canada.
Submitted in: Journal of Molecular Liquids
All DFT simulations and analysis along with the paper have been written and presented by Dariush Azizi. The scientific revision was done by Prof. Faïçal Larachi.
Besides, some of the research results were presented in the following conference:
D. Azizi, F. Larachi, Ionic-liquid self-aggregation on rare-earth semi-soluble mineral surfaces
in aqueous and non-aqueous systems, 66thCanadian Chemical Engineering Conference, Quebec,
Canada, 2016.
Moreover, some of the research results were presented in poster form in the following conferences:
A. Sarvaramini, D. Azizi, F. Larachi, Hydroxamic acid interactions with solvated cerium
hydroxides in the flotation of monazite and bastnäsite—Experiments and DFT study, 7e
1.1. State of Technology
In recent years, the rare earth elements (REEs) have been attracting considerable attention particularly owing to their application in critical technologies and also concerns over their supply security [1]. Their significance was more evident when the European Commission and also the US Department of Energy (DOE) considered REEs as critical elements and started to prepare long-term strategies against supply interruptions [2]. This perspective was more pronounced since 2009, when China applied protectionist policies to limit yearly export quotas, while the world demand has been increasingly increased [2-4]. In order to mitigate this problem, alternatives such as recycling of materials containing REEs (as secondary source of REEs) and more importantly development of new REEs mining projects (as primary source of REEs) have been put in place by other countries [3, 4]. However, these new mining projects are always encountering new challenges as each REEs deposit has its own unique specificities [5]. This means that REEs production from REE-bearing minerals is a critical subject that needs further studies regarding involved processes such as froth flotation, leaching and also liquid-liquid REEs extraction. In this optics, introducing new effective chemical reagents applicable in flotation, leaching and liquid-liquid extraction and then determining their behavior and interaction mechanisms in the corresponding processes are necessary.Improvement of these processes in REEs processing depends mainly on the development of chemical reagents. In the last decade, ionic liquids (ILs) have been the focus of considerable interest for the development of environmentally friendly solvent extraction systems for various metals and in particular for REEs separation [6-8]. ILs have shown that they can be used as either diluents or extractant in solvent extraction systems [6]. Likewise, those ILs with both moieties are functionalized towards metals extraction, can even outperform conventional extractants [6]. In addition, ILs can be generally considered as green solvents thanks to their high-thermal stability, very low flammability, and negligible vapor pressure. Therefore, the tendency towards use of ILs in REEs processing has been expanding considering the fact that in recent years, awareness of relying on “green chemistry” has also become widespread [6].
In spite ILs have already been shown to exhibit features as extractant or solvent through liquid-liquid REEs extraction, they have not been sufficiently explored as chemical reagents in flotation REE minerals. Due to their potential and selectivity in liquid-liquid extraction and as there are many similarities involved in chemical needs in flotation, leaching, and solvent extraction, ILs could be considered as an effective alternative to REE minerals treatment. Unfortunately, there is the lack of information and a significant gap in the area of REE minerals treatment using ILs as the chemical reagents. It indicates that there is room for research opportunities in REEs production industry considering ILs’ technologies.
The aim of this section is to highlight the current knowledge and problems in REEs processing, and also to discuss about the current status and potential of ILs in minerals and metal processing. By taking into account these two subjects, one may conclude that ILs could be considered as potential chemical reagents to improve efficiency of REE minerals processing industry in the future.
1.2. Rare Earth Elements
1.2.1. Natural Sources, Supply and Possible Problems
The REEs designation was originally customized to refer to a group of 17 metallic elements of the periodic table, namely, the lanthanide group fifteen elements, and also yttrium and scandium. These elements are divided according to their atomic mass into two main groups, i.e., light REEs (LREEs) and heavy REEs (HREEs) [9, 10-12]. Either HREEs or LREEs are needed and extensively utilized for various applications in industry. However, their supply and availability are always under attention [1, 3, 13, 14]. Natual occurrence of REEs consists of 250 different REE-bearing minerals comprising silicates, oxides, carbonates, phosphates and halides [4, 9, 15]. The most important naturally-occurring REE-bearing minerals are bastnäsite ((REE)FCO3), monazite ((REE)PO4) and xenotime
(YPO4). Mountain Pass in the US and Bayan Obo in China for bastnäsite, Mount Weld in Australia
and Green Cove Spring in the US for monazite, Lehat in Malaysia for xenotime [4], and St-Honoré [16] in Canada for ores with monazite and bastnäsite, are examples of primary deposits of these minerals.
Fig.1-1 and Table1-1, respectively, list the most important countries involved in the production of REEs oxides in 2014 and also the average price of selected REE oxides (March 2015) [14]. China is considered as the primary supplier of these elements and is playing a significant role in the market [1, 13]. Also, the price of HREEs is larger than that of LREEs and this should always be taken into consideration to develop production processes.
Availability of REEs may be at risk due to factors such as single-country control of REEs supply, and also individual REEs are not mined separately. Hence, studies have predicted that application and demand of REEs keep growing forecasting for future availability to be critical for industry [1].
Table 1-1: The average price of selected rare earth oxides (March 2015) [14].
China domestic market Price (US $ /Kg)
Lanthanum oxide 1.95 Cerium oxide 1.8 Praseodymium oxide 59.85 Neodymium oxide 46.5 Samarium oxide 2.55 Europium oxide 260.4 Gadolinium oxide 12.9 Terbium oxide 606.6 Dysprosium oxide 276.6
Fig.1-1: Most important countries in rare earth production (2014) [14].
Another critical issue in REEs production is the environmental concerns regarding their mining and processing which have always been pointed out. Most of the REE deposits are associated with high concentration of radioactive elements such as uranium and thorium. These radioactive elements may be accumulated in tailing dams and thus haphazardly introduced into the environment. There are examples which can be noted to reveal the negative environmental impact of REEs processing. The most important is related to the radioactive pollution of Asian Rare Earth Company (Malaysia) and also of the Mountain Pass (US) projects. Besides, the REEs production always is also known for its gullibility for important water volumes, chemical reagents and energy consumption [13, 17, 18]. Therefore, despite the fact that the development of new mining projects may be considered as a solution for REEs supply shortages, environmental concerns during REEs production should be taken into account.
1.2.2. Specific Properties of Rare Earth Elements
One important characteristic of REEs is the filling up of their 4f subshell by electrons which causes gradual reduction in their ionic radius (as seen in Fig.1-2). This behavior is known as the lanthanides contraction [11, 12]. It simply is the consequence of increasing their nucleus charges resulting in electron shells being pulled closer to the atom’s nucleus. According to this behavior, the stability of REE complexes varies considering their atomic numbers. Differences in their complex stabilities have been regarded as a useful property to exploit in the separation of individual lanthanides in a state of high purity [11, 19]. In addition, their metal reactivity increases gradually from scandium to lanthanum with the latter considered as the most reactive among REEs [12].
0 10 20 30 40 50 60 70 80 90 100 86.3 6.3 2.2 2.7 2.2 1 0.2 0.2 P ro du ct io n pe rc en ta ag e
Fig.1-2: Reduction in ionic radius of REEs by increasing their atomic number [10].
These elements are considered as strong Lewis acids and tend to yield complexes with atoms belonging to the hard base groups [12]. These elements mostly tend to make the complex with O, but other kinds of complexes for these elements are observed as well. The abundance of their complexes with various atoms follows this sequence: RE–O > RE–N > RE–C > RE–S > RE–P > RE–Te > RE– Se > RE–Si. This feature of REEs is employed to find appropriate chelating agents for their extraction [10, 11].
The lanthanide tetrad effect. first introduced in 1969 [21] [22, 23], is considered as another observed important phenomenon arising due to the fill up of electrons into their 4f shell (likening lanthanides contraction) [10, 20]. According to this -effect, REEs are categorized into four groups (lanthanum– cerium–praseodymium–neodymium, promethium–samarium–europium–gadolinium, gadolinium– terbium–dysprosium–holmium and erbium–thulium–ytterbium–lutetium) considering their behavior in various extraction systems. The discovery of the lanthanide tetrad effect has been used in the separation of lanthanide elements through liquid-liquid extraction [10, 20]. Fig.1-3 demonstrates that both lanthanide tetrad effect and contraction have significant effects on REEs separation which could be taken into consideration through solvent extraction of REEs.
Fig.1-3: Relationship between atomic number of lanthanides and thermodynamic functions (Kex
extraction equilibrium constant, ΔH enthalpy variation, ΔZ0r free energy variation, and ΔS0r entropy variation) of an extraction reaction from a system consisting of 2-ethyl hexyl mono (2-ethyl hexyl) ester phosphinate in a dodecane solution [10].
1.3. Rare Earth Bearing Minerals Beneficiation
Enrichment processes of REE minerals consist of various mineral processing methods such as gravity, magnetic and electrostatic separations, and specially froth flotation. Among all these enrichment methods, froth flotation is the most common and applied one which is highly dependent on various chemical reagents [4, 9]. Hence, we will discuss the chemistry of REEs minerals flotation to show up demands and opportunities for further development of new chemical reagents. Besides, since bastnäsite and monazite are known as the most abundant of REE minerals and so targeted REEs minerals of this study, the discussion will be focused only on their flotation.
1.3.1. Rare Earth Bearing Minerals Flotation Difficulties
Froth flotation has been predominantly employed to beneficiate REE minerals owing to its unique process features to handle a range of particle sizes and composition and being versatile vis-à-vis the mineralogical characteristics of any given deposit [4, 9]. Despite that fact that froth flotation is most commonly applied method, it is not exempt from challenges. The first most significant one encountered in REE minerals flotation reflects in discrepancies in REE minerals composition associated with similarity from the surface chemistry standpoint of REE minerals and their associated
gangue minerals [24, 25]. The second one is related to the inadequate response of froth flotation to fine REE mineral particles as a general drawback of mineral flotation. It occurs owing to changes in the mineral surface features, physicochemical and hydrodynamic conditions [26-28]. This problem is particularly acute in REE minerals flotation as large amounts of fine particles are produced during grinding [29]. In fact, REE minerals usually need fine grinding to meet enough liberation for flotation due to high association with their gangue minerals. However, they are easily slimed during grinding process because of their brittleness [29]. To get rid of these two main REE minerals flotation problems, some researchers have attempted to develop new methods such as the use of reactive oily bubbles [30], flocculants [29] and also liquid-liquid extraction [31, 32] to beneficiate these minerals. Besides, many investigations have focused on further understanding of REE minerals flotation aimed at improving REE minerals flotation. In this regard, studies on the effectiveness of flotation reagents and also on the surface chemistry of REE minerals have been numerous. Although many attempts to improve knowledge about surface and regent chemistry of REE minerals flotation, further studies in these areas are still required for bettering our understanding and for improving REE minerals flotation [4, 24].
1.3.2. Surface Chemistry of Rare Earth Bearing Minerals
The response of flotation of REE minerals is highly dependent on their surface charges, zeta potential, solution chemistry and surface species [17, 25]. Further understanding about these parameters could contribute to the flotation improvement. The electrokinetic behavior of bastnäsite and monazite as semi-soluble salt minerals is related to their potential-determining ions (PDIs) [25, 33]. For bastnäsite, H+, OH- and CO
32- and for monazite H+, OH- and PO43-are considered to determine their surface
potentials. Recently, REEs have been introduced as other PDIs for both minerals [25, 33]. The point of zero charges (PZC) and the isoelectric point (IEP) are critical parameters for a better understanding of the surface chemistry of minerals [34].Various IEPs have been reported with pH ranges from 1.1 to 9.0 for monazite and 4.6–9.5 for bastnäsite [4, 17]. These variations in IEP could be due to changes in chemical composition of minerals from different deposits, differences in the presence of impurities and crystal structure and differences in experimental conditions [17, 24]. Furthermore, monazite and bastnäsite exhibited differences in their IEP values which were ascribed to their different potential-determining conjugate-base ions, i.e., CO32- and PO43- [25, 33]. Carbonic acid being weaker than
phosphoric acid, to reach equilibrium electrokinetic state on monazite surface, this latter’s IEP is expected to shift towards lower pH than bastnäsite [25].
Studies on speciation of bastnäsite in aqueous solution revealed that bastnäsite is the only solid phase in the pH range from 5.75 to 8.55. Bastnäsite coexists with CeF3(s) from pH 5.22 to pH 5.74 and
with Ce(OH)3(s) from pH 8.56 to pH 10.12. Cerium fluoride and cerium hydroxide are the only solid
phases below pH 5.2 and above pH 10.2, respectively [35]. Some researchers considered only REEPO4as the solid phase for monazite in aqueous solution over the entire range of pH [36]. Studies
on their wetting characteristics indicated that either monazite or bastnäsite are highly hydrophilic due to greater water density distribution profiles of the former [25].
1.3.3. Collectors in Rare Earth Bearing Minerals Flotation
Froth flotation is a process of separation and concentration of one kind of minerals from other ones based on differences of their hydrophobicity [37].Wettability of REE minerals is almost the same as their associated gangue. Therefore, REE minerals should be selectively made hydrophobic by reagents for their beneficiation [38]. For this purpose, various collectors are extensively applied in their flotation. Collectors are a group of organic chemicals with varying chemical compositions and functional groups. They aim is to selectively form a hydrophobic layer on the surface of a target mineral thus providing the necessary hydrophobicity for attachment to air bubbles [39].
Selection of an appropriate collector is crucial through REE minerals flotation, owing to their complex flotation. Hence, in recent years, numerous studies have become available to identify collectors with high selectivity and collecting power [38]. Collector uptake by REE minerals occurs through two primary mechanisms namely, surface reaction-precipitation and chemisorption. Through chemisorption reaction (less important mechanism), a collector directly interacts with an active center (metal cation) on the mineral surface resulting in a monolayer of surfactant ions. The interaction of semi-soluble salt minerals such as REE minerals with chelating or complexing agents mostly occur by surface reaction-precipitation [17, 37, 40]. This interaction is facilitated firstly by the dissolution and hydroxylation of metal cations from lattice sites, and then re-adsorption of the hydroxylated metal cations on the mineral surfaces. These hydroxylated species on the mineral surface act as active centers when chelation occurs. These two steps are highly dependent on pH since a variety of REE species occur over the entire range of pH. Besides, this chelation interaction may occur in the mineral-aqueous phase interface and then complexes precipitate on the mineral surfaces [17, 24, 25, 36, 40, 41]. The effectiveness of various anionic collectors bearing carboxylic, hydroxamic and phosphorous acid functional groups, towards bastnäsite and monazite REE minerals has been studied recently [4, 17, 24, 25, 38, 42, 43]. Owing to their low cost and large availability, various fatty-acid carboxylic group-containing collectors such as sodium oleate, oleic and phthalic acids have been used for the flotation of bastnäsite and monazite [24, 43]. Sodium oleate, for instance, was found to outperform oleic acid in terms of floatability [17]. Also, phthalic acid, a dicarboxylic acid with benzene ring, exhibited superior affinity to bastnäsite as compared with monazite to separate them effectively [43]. Likewise, benzoic acid enabled separation of monazite from bastnäsite by adding potassium alum as a depressant for monazite [44]. Besides, tall oil fatty acids (Aero 704, Sylfat FA2) as collectors have been used in the flotation of xenotime, monazite-(Nd) and REE carbonates where they were associated with silicate minerals and hematite [45]. This investigation indicated that liberation of REE minerals can be considered as an important parameter through flotation by this family of collectors. The mechanism purported in the uptake for this collector is chemisorption between its carboxyl group and predominantly hydroxylated active-center REEs and metal cations of the mineral surface [17, 24, 36, 46]. Following COOH deprotonation, the carboxylate conjugate-base anion is thought to interact possibly with the mineral surface via nascent bonds of its carbonyl group (C=O), of its deprotonated hydroxyl group or of both [47]. Monolayer chemisorption of this collector on bastnäsite and monazite is endothermic pointing to the fact that flotation selectivity improves with increasing temperature [24]. However, for achieving in practice sufficient selectivity for this collector, high depressant concentrations, and elevated temperatures are used [24]. This has prompted many investigations in quest of new collectors with sufficient selectivity.
As a result of such efforts, derivatives of both hydroxyl amine and carboxylic acids exemplified in hydroxamic acid-containing collectors have been found as an effective alternative [48]. A specific feature of such oxyhydryl collectors is their ability to form metal coordination complexes by acting as bi-dentate ligands with various metal cations, such as transition, lanthanide and actinide metal ions [47]. Because of higher stability of the hydroxamate–metal complexes formed with REE cations at the surface of bastnäsite and monazite, these collectors showcase superior flotation selectivity of REE minerals in comparison with fatty acids [17, 24]. The increased stability of hydroxamic acid-containing collectors versus fatty acid ones stems from the presence, in the former, of a more electronegative nitrogen atom. Also, in comparison with alkaline earth metals, which are present as associated gangue minerals, REE cations are preferential coordination centers for hydroxamate complex formation [47]. It is also recently confirmed that hydroxamate is selective to Ce over Ca with the aid of a novel model system for investigation of REE minerals flotation [49]. The collector’s interaction with bastnäsite and monazite has been recognized to manifest as a chemisorption in which hydroxylated REEs re-adsorb at the mineral surface prior to interact with hydroxamate through anion exchange to form stable REE surface complexes [40, 46, 50, 51]. Moreover, it has been reported that the redox chemistry plays a major role in the superior beneficiation of bastnäsite using an alkyl hydroxamic acid-containing collector over other flotation collectors such as fatty acids. For instance, oxidation of cerium III to IV was alleged to improve adsorption of hydroxamic acid-containing collectors on bastnäsite surface as a result of Ce(IV) stabilization [52].
Various hydroxamate collectors are in use for the flotation of bastnäsite and monazite. Alkyl hydroxamic acid-containing collectors (RCONHOH), whose adsorption is pH and temperature sensitive [25, 40, 41, 53], are widely used in monazite and bastnäsite flotation in conjunction with reagents such as sodium carbonate, sodium silicate and sodium fluorosilicate added as regulators, depressants, and modifiers [17, 24]. As an example, this collector proved highly selective to separate bastnäsite from calcite and barite. Benzohydroxamic acid (C6H5CONHOH), a sibling collector from
the same family, has also been used for the flotation of REE minerals, especially HREEs bearing minerals [54]. Its performance has been shown to depend on the selected frother while enabling eventually to skip the de-sliming step [24]. Contrary to their alkyl analogs, cycloalkyl hydroxamic acids (CnH2n-1CONHOH), due to their lower aqueous solubility and hydrolizability, have been less
effective in REE minerals flotation even aided with modifiers [43]. Finally, salicylic hydroxamic acid (C6H4OHCONHOH) used in combination with sodium silicate depressant enabled promising
high-grade REE minerals concentrate to be obtained [43, 54]. Despite the fact that hydroxamic acid collectors have demonstrated higher selectivity than carboxylic acids bearing collectors, the hydroxamic acids are still not ready as commercial chemicals, because of cost and quantities required for flotation [19].
Organo-phosphorous acids derived from phosphoric ((OH)3PO), phosphonic ((OH)2HPO) and
phosphinic ((OH)H2PO) acids represent another type of anionic collectors for REE minerals [24, 38,
47]. Phosphorous acids are much more acidic than hydroxamic and carboxylic acids foreseeing superior performance of phosphorous-containing collectors in the lower pH range. P-bearing collectors are able to form complexes with various metal cations via the two O atoms tethered in their functional group [47]. Alkyl phosphate ester collectors have been used for the separation of bastnäsite from monazite with the help of citric acid as a regulator [43]. Citric-acid-mediated separation by these collectors of REE minerals from silicates led to promising results [55]. Even though the industrial
![Fig. 2-4: Micro-flotation tests of model minerals with Aero-6493 and [N 4444 ][DEHP] (pH 9, IL and collector concentration = 100 mg/L)](https://thumb-eu.123doks.com/thumbv2/123doknet/5003100.124495/77.918.133.790.169.471/micro-flotation-tests-model-minerals-aero-collector-concentration.webp)
![Fig 2-12: High-resolution XP P 2p core level spectra along with fitted features for pure [N 4444 ][DEHP] (a), bastnäsite samples recovered after flotation after adsorption of [N 4444 ][DEHP]](https://thumb-eu.123doks.com/thumbv2/123doknet/5003100.124495/86.918.132.791.167.758/resolution-spectra-features-bastnäsite-samples-recovered-flotation-adsorption.webp)
![Fig. 2-14: Speculated adsorption pathway of [N 4444 ][DEHP] on bastnäsite and monazite surfaces at 7 < pH < 9.](https://thumb-eu.123doks.com/thumbv2/123doknet/5003100.124495/88.918.133.793.299.830/fig-speculated-adsorption-pathway-dehp-bastnäsite-monazite-surfaces.webp)